Prescribing - achieving value and sustainability - draft guidance: consultation
We are consulting on draft guidance on achieving value and sustainability in prescribing by reducing unwarranted variation across health boards. This includes, items of low and limited clinical value, diabetic sundries, and antimicrobial stewardship.
4. Achieving Value and Sustainability in Prescribing
Executive Summary
This guidance aims to reduce the use of low value medicines and ensure the effective use of medicines with limited clinical value throughout NHS Scotland. In addition, it aims to ensure best value in the choice of blood glucose monitoring and insulin pen needles and to ensure the most effective use of antimicrobials (antibiotics) to minimise the risk of antimicrobial resistance. It provides guidance for NHS Health Boards and clinicians, to minimise the unwarranted variation in the prescribing of medicines and make the most efficient use of resources and supports the principles of Realistic Medicine through shared decision making, encouraging medicine review, and ensuring medicines are not prescribed inappropriately.
This guidance was produced by an expert working group of the Value and Sustainability Prescribing Group and is predominately based on the NHS England policy guidance: Items which should not routinely be prescribed in primary care and the NHS Wales Medicines identified as low priority for funding in NHS Wales documents. It also includes items which have been requested by Health Boards within NHS Scotland.
Delivering value based health and care: a vision for Scotland defines value-based health and care (VBH&C) as the equitable, sustainable, and transparent use of resources to achieve better outcomes and experiences for every person in Scotland. The aim is to reduce unwarranted variation and maximise the value of healthcare, to achieve the outcomes that matter to the individual and to lead to a more sustainable health care system. NHS Scotland has finite resources; therefore, it is important to ensure their optimal use to reduce waste, harm, and unwarranted variation.
Across Scotland there is significant variation in what is prescribed and to whom, and there are often more effective, safer, or cost-effective alternatives available. Good prescribing dictates that the choice of therapy should be made in discussion with the individual and on the basis of good clinical evidence of efficacy, safety, value and acceptability. It is good practice to prescribe the best value preparation available for the most appropriate duration, and to prescribe solid dosage forms where appropriate for cost, environmental and safety reasons. For example, effervescent analgesic preparations are high in salt content and often more expensive than standard tablets.
Cost-effectiveness of Diabetic Sundries
Included in a subsection of the limited value medicines in this guidance, are insulin pen needles and blood glucose monitoring strips. Although clinically effective these have been included as they offer low value to the NHS above the cost limits stated as there are a variety of more cost-effective alternatives available, e.g. insulin pen needles costing more than £5 for 100 needles.
Anti-microbial Stewardship
A further section of this guidance promotes the safe, effective and sustainable use of antimicrobials. Although antimicrobials are medicines of high clinical value they can be of less value when prescribed inappropriately, e.g. where not clinically indicated (viral infection), or when prescribed for longer than their recommended duration.
The aim of this section is to provide recommendations on the effective prescribing of antimicrobials. It complements the work of the Scottish Antimicrobial Prescribing Group (SAPG), who promote the safe and effective use of antibiotics across hospital and community settings to improve antibiotic stewardship and tackle the risks of antimicrobial resistance. It includes recommendations on the appropriate use of antibiotics, appropriate duration and advice on prescribing solid oral dosage forms instead of liquid or injectable preparations. These will minimise both environmental and financial cost.
Prescribers are encouraged to have due regard for this guidance when considering prescribing the items included. However, it does not remove the clinical discretion of the prescriber in discussing and agreeing the most suitable treatment for individuals in accordance with their professional duties and to minimise harm or unintended consequences.
4.1 Items of Low and Limited Clinical Value
Recommendations
The recommendations for medicines of low and limited clinical value are grouped under two categories:
1. Items where no prescribing is appropriate (no exceptions apply) – low clinical value
2. Items where prescribing may be appropriate in exceptional circumstances – limited clinical value.
The following policy recommendations apply to all items in the low value category:
- do not initiate
- consider deprescribing where safe in individuals currently prescribed this item
The following recommendations apply to items in the limited value category:
- prescribe only if the item is for an indication named in this guidance
- prescribe only if no other item or intervention is clinically appropriate
- prescribe only if no other item or intervention is available
Prescribing Guidance for Medicines of Low or Limited Clinical Value
Items of low clinical value
Items where no prescribing is appropriate because there are significant safety concerns, no evidence of clinical effectiveness or no additional benefits compared with cost-effective alternatives.
The total spend for NHS Scotland was £3,847,247 for these medicines in the financial year 2022/23.
Recommendations
- do not initiate in primary or secondary care
- deprescribe safely in individuals currently prescribed this item
These recommendations apply to:
- co-proxamol
- glucosamine and chondroitin
- herbal treatments
- homeopathy
- minocycline for acne
- aliskiren
- bath and shower emollients
- dipipanone hydrochloride and cyclizine
- doxazosin modified release preparation
- lutein and antioxidants
- nefopam
- omega-3 fatty acids excluding Vazkepa®
- oxycodone and naloxone combination product
- paracetamol and tramadol combination product
- perindopril arginine
- probiotics
- rubefacients and poultices (excluding capsaicin)
Co-proxamol
Recommendation:
- Do not initiate in primary or secondary care
- Deprescribe in individuals currently prescribed this item
Exceptions:
- None
Background and rationale for recommendation:
- Co-proxamol was fully withdrawn from the UK market in 2007 by the Medicines and Healthcare products Regulatory Agency (MHRA) due to safety concerns regarding toxicity and risk of fatal overdose.
- There is no licensed preparation available in the UK therefore any existing prescribing is on an unlicensed basis
- The cost of prescribing co-proxamol as an unlicensed ‘special’ is significantly higher than the licensed cost-effective alternatives as it requires to be imported for individual use at a cost to the NHS and the environment.
- The paracetamol dose in each tablet is at a lower dose (325mg) than in standard paracetamol preparations (500mg).
- There is no robust evidence that co-proxamol is more effective than full strength paracetamol.
- There is a risk of addiction and abuse associated with co-proxamol.
- Co-proxamol is potentially fatal in overdose. The lethal dose of is relatively low and can be potentiated by alcohol and other central nervous system depressants
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual spend:
- £96,043 (2022/23)
Glucosamine and Chondroitin
Recommendation
- Do not initiate in primary or secondary care
- Deprescribe in individuals currently prescribed this item
Exceptions:
- None
Background and rationale for recommendation
- Glucosamine and chondroitin supplements are used to improve pain associated with arthritis and can be purchased from pharmacies, supermarkets, and health food stores.
- Glucosamine is deemed less suitable for prescribing by the British National Formulary (BNF) as the mechanism of action is not understood and there is limited evidence to show it is effective.
- NICE guidance on osteoarthritis care and management recommends: Do not offer glucosamine or chondroitin products for the management of osteoarthritis.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £1025 (2022/23)
Herbal treatments
Recommendation
- Do not initiate in primary or secondary care
- Deprescribe in individuals currently prescribed this item
Exceptions:
- None
Background and rationale for recommendation
- There is a lack of robust evidence of the clinical effectiveness of herbal treatments. Herbal treatments come in a variety of formulations, such as tablets, capsules, powders, and sprays. and can be bought over the counter.
- There is a lack of scientific evidence required to register these products with the MHRA. Under a traditional herbal registration (THR) there is no requirement to prove scientifically that a product works; the registration is based on longstanding use of the product as a traditional medicine.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £236 (2022/23)
Homeopathy
Recommendation
- Do not initiate in primary or secondary care
- Deprescribe in individuals currently prescribed this item
Exceptions:
- None
Background and rationale for recommendation
- Homeopathy is a complementary or alternative therapy that seeks to treat individuals with highly diluted substances that are administered orally. Homeopathy is mainly available in tablet form but also comes in drops, capsules, and powders.
- These items can be bought over the counter.
- There Is a lack of robust evidence for its clinical effectiveness.
- The specialist pharmacist service (SPS) reviewed the evidence for homeopathy for the NHS England ‘Items which should not be routinely prescribed in primary care’ consultation.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £90,570 (2022/23)
Minocycline for acne
Recommendation:
- Do not initiate for treatment of acne
- Deprescribe in individuals currently prescribed this item for treatment of acne.
Exceptions:
- None
Background and rationale for recommendation
- Minocycline is a tetracycline antibiotic that is mainly prescribed for acne in primary care but does have other indications. There are various safety risks associated with its use.
- The BNF states that minocycline is less suitable for prescribing compared with other tetracyclines, as it is associated with a greater risk of lupus-erythematosus-like syndrome, and sometimes causes irreversible pigmentation
- And if treatment is continued for longer than 6 months, monitor every 3 months for hepatotoxicity, pigmentation and for systemic lupus erythematosus.
Category:
- Items of low clinical effectiveness in acne, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £60,877 (2022/23) – includes all uses of minocycline
Aliskiren
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Aliskiren is a renin inhibitor which inhibits renin directly. It is indicated for essential hypertension either alone or in combination with other antihypertensives.
- Scottish Medicines Consortium (SMC) advice states that aliskiren (Rasilez®) is not recommended for use within NHS Scotland for the treatment of essential hypertension. Aliskiren has shown comparable efficacy to other antihypertensive agents in terms of blood pressure reduction, though its effects on mortality and long-term morbidity are currently unknown.
- The Medicines and Healthcare products Regulatory Agency (MHRA) in 2014 recommended caution with aliskiren following the ALTITUDE study for the following patients:
- Patients taking aliskiren in combination with an ACE inhibitor or an ARB.
- Patients with severe renal impairment—ie. eGFR <30mL/min per 1.73 m2
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £15,433 (2022/23)
Bath and shower emollients
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Emollient bath and shower preparations are routinely prescribed for dry and pruritic skin conditions including eczema and dermatitis.
- The BATHE randomised control trial: Adding emollient bath additives to standard eczema management for children with eczema showed that there was no evidence of clinical benefit for including emollient bath additives in the standard management of childhood eczema.
- Although the evidence from the BATHE study applied only to children, it was agreed to extrapolate this to use in adults whilst awaiting further evidence.
- These preparations can leave an oily residue on the skin, bath or shower which can increase the risk of falls.
- ‘Leave-on’ emollient moisturisers are still recommended for the management of eczema and can be used as soap substitutes.
- The specialist pharmacist service (SPS) reviewed the evidence for bath and shower emollients for the NHS England ‘Items which should not be routinely prescribed in primary care’ consultation
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of increased effectiveness or significant safety concerns..
Annual Spend:
- £1,224,332 (2022/23)
Dipipanone hydrochloride and cyclizine
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Dipipanone is a strong opioid analgesic drug, used for acute pain and is only indicated for short term use.
- There is no evidence to suggest that dipipanone & cyclizine offers any additional clinical benefit or is superior to alternative opioids.
- Dipipanone is a short acting opiate. The short acting effects of both dipipanone and cyclizine increase the risk of both physical and psychological dependency.
- Diconal® which was the branded version of dipipanone and cylizine was discontinued in 2011. The cost is now 40 times more expensive - £440.65 for 50 tablets versus £9.57 for 50 tablets.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of increased effectiveness or significant safety concerns.
Annual Spend:
- £27,549 (2022/23)
Doxazosin modified release (MR) preparation
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Doxazosin is an alpha-adrenoceptor blocking drug that can be used to treat hypertension and benign prostatic hyperplasia. It is available as a modified release and an immediate release preparation (IR), both of which are taken once daily.
- The long half-life of the IR preparation allows for once daily dosing and the MR preparation offers no advantage in efficacy in comparison.
- MR doxazosin is approximately six times the cost of IR Doxazosin.
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £337,878 (2022/23)
Lutein and antioxidants
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Lutein and antioxidants (e.g. vitamin A, C, E, and zinc) are supplements recommended for age-related macular degeneration and are not licensed medicines. These items can be bought over the counter and in health food stores.
- PrescQIPP CIC has issued a bulletin which found no evidence to support routine prescribing of Lutein and antioxidants.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or there are significant safety concerns.
Annual Spend:
- £623,097 (2022/23)
Nefopam
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Nefopam is a centrally acting non-opioid analgesic with associated antimuscarinic and antihistaminergic effects recommended for persistent pain unresponsive to other non-opioid analgesics. It is indicated for the relief of acute and chronic pain, including post-operative pain, dental pain, musculoskeletal pain, acute traumatic pain, and cancer pain.
- The 2019 revised edition of the SIGN guideline “Management of Chronic Pain” identified insufficient evidence on the use of nefopam for chronic pain relief to support a recommendation.
- The Specialist Pharmacy Service (SPS):Use of nefopam for chronic pain review found no evidence that nefopam is more potent than non-steroidal anti-inflammatory drugs (NSAIDs), but that it is commonly associated with adverse drug reactions and is toxic in overdose.
- Nefopam scores 2 on the anticholinergic burden scale (ACB) therefore sympathomimetic and antimuscarinic side-effects may be troublesome.
- Adverse effects are common and include nausea, sweating, dizziness, vomiting, hallucinations, confusion, urinary retention, headache, insomnia, tachycardia, palpitations convulsions and anaphylaxis.
- When abused, nefopam has primarily psychostimulant-like effects, which are probably linked to its dopamine reuptake inhibition properties.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or there are significant safety concerns.
Annual Spend:
- £478,865 (2022/23)
Omega-3 fatty acids excluding icosapent ethyl (Vazkepa®)
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Omega-3 fatty acid compounds are essential fatty acids which can be obtained from the diet. They are licensed for adjunct to diet and statin in hypertriglyceridemia; adjunct to diet in type IV hypertriglyceridemia. Omega-3 fatty acid compounds can be bought over the counter.
- A recent MHRA drug safety update highlighted a dose-dependent increased risk of atrial fibrillation in individuals with established cardiovascular diseases or cardiovascular risk factors taking Omega-3-acid ethyl ester medicines (Omacor®/Teromeg® 1000mg capsules).
- Omega-3-acid ethyl esters (Omacor®) is not recommended by the SMC for use within the NHS Scotland for hypertriglyceridaemia.
- There is no good quality data for the use of omega-3 fatty acid compounds in prevention of dementia, pre-menstrual syndrome, attention-deficit hyperactivity disorder (ADHD), atrial fibrillation, eczema, osteoarthritis, or age-related macular degeneration.
- Individuals currently prescribed omega-3 fatty acids should be reviewed. Acute pancreatitis can be precipitated on withdrawal in those prescribed omega-3 fatty acids for disorders of triglyceride metabolism, therefore, any review in these individuals should be made in conjunction with relevant specialist teams.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or there are significant safety concerns.
Annual Spend:
- £260,613 (2022/23)
Oxycodone and naloxone combination product
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Oxycodone and naloxone combination product is used to treat severe pain and can also be used second line in the treatment of symptomatic severe to very severe idiopathic restless legs syndrome after failure of dopaminergic therapy. The opioid antagonist naloxone is added to counteract opioid-induced constipation by blocking the action of oxycodone at opioid receptors locally in the gut.
- SMC advice states that oxycodone/naloxone prolonged release tablets are not recommended for use within NHS Scotland for the treatment of severe pain which can be adequately managed only with opioid analgesics. The addition of naloxone to oxycodone did not impair analgesia and improved bowel function when individuals were not receiving regular laxative therapy. However, the clinical benefit in individuals receiving regular laxative therapy is uncertain.
- A PrescQIPP bulletin Oxycodone/naloxone (Targinact®) did not identify a benefit of oxycodone and naloxone combination product over other analgesia (with laxatives if necessary).
- The cost of the combination product is significantly more expensive than alternative opioids.
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £203,851 (2022/23)
Paracetamol and tramadol combination product
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Paracetamol and tramadol are both commonly available analgesics. This recommendation relates to where both chemical ingredients are used together in a single combination product (tramacet®).
- The SMC has advised that tramadol 37.5mg/paracetamol 325mg tablet (Tramacet®) is not recommended for use within NHS Scotland for the symptomatic treatment of moderate to severe pain.
- Tramacet® costs significantly more than its individual components prescribed separately.
- No evidence that the combination product is more effective or safer than the individual preparations and it contains a sub-therapeutic dose of paracetamol
- Paracetamol and tramadol combination products are more expensive than the products with the individual components.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence or significant concerns.
Annual Spend:
- £19,379 (2022/23)
Perindopril arginine
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Perindopril arginine (coversyl®) is an angiotensin-converting enzyme inhibitor (ACEi) indicated for use in the treatment of hypertension, treatment of symptomatic heart failure for reduction of risk of cardiac events in individuals with a history of myocardial infarction and/or revascularisation.
- The perindopril arginine salt version was developed as it is more stable in extremes of climate than the perindopril erbumine salt, which results in a longer shelf-life however in the context of the UK climate and supply chain this benefit is not significant.
- Perindopril arginine is more expensive than perindopril erbumine and a PrescQIPP bulletin stated there was no clinical advantage of the arginine salt.
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £97,892 (2022/23)
Probiotics
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Probiotics are live micro-organisms that are thought to restore the natural balance of bacteria in the gut.
- There is currently insufficient clinical evidence to support prescribing of probiotics within the NHS for the treatment or prevention of diarrhoea of any cause.
- The Advisory Committee on Borderline Substances (ACBS) reviewed the probiotic products VSL#3® and Vivomixx™ and concluded that the evidence available did not sufficiently demonstrate that the products are clinically effective resulting in their removal.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £75,849 (2022/23)
Rubefacients and poultices (excluding capsaicin)
Recommendation:
- Do not initiate in primary or secondary care.
- Deprescribe where safe in individuals currently prescribed this item..
Exceptions:
- None
Background and rationale for recommendation
- Rubefacients are topical preparations that may contain nicotinate compounds, salicylate compounds, essential oils, and camphor. They cause irritation and reddening of the skin due to increased blood flow. They are believed to relieve pain in various musculoskeletal conditions and are available on prescription and in over-the-counter remedies.
- The BNF states: “The evidence available does not support the use of topical rubefacients in acute or chronic musculoskeletal pain”.
- NICE has recommended: Do not offer rubefacients for treating osteoarthritis.
- A Cochrane review found the evidence does not support the use of topical rubefacients containing salicylates for acute injuries or chronic conditions.
Category:
- Items of low clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £233,758 (2022/23)
Items of limited clinical effectiveness, where prescribing may be appropriate in some exceptional circumstances
For all items, if no other item is clinically indicated or available, it may be appropriate to prescribe either through specialist initiation or in some cases following a shared decision-making conversation between the prescriber and individual. This should be decided using good quality evidence, clinical judgement and the individual’s values and preferences.
Recommendations
- prescribe only if for an exception or indication named in this guidance
- consider deprescribing in individuals currently prescribed this item where appropriate
- prescribe only if no other item or intervention is clinically appropriate
- prescribe only if no other item or intervention is available
These recommendations apply to:
- alimemazine
- amiodarone
- ascorbic acid
- buprenorphine patches
- chloral hydrate/chloral betaine
- dosulepin
- dronedarone
- immediate release fentanyl
- lidocaine plasters
- liothyronine
- trimipramine
Alimemazine
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- As a premedication to anaesthesia in children two to six years old.
Background and rationale for recommendation
- Alimemazine is a first generation H1 sedating antihistamine and is a phenothiazine derivative. It is licensed in children aged three to seven years for pre-medication sedation before general anaesthesia and in adults and children aged three years and over for second-line treatment in the symptomatic relief of urticaria and pruritus.
- No evidence available to state that alimemazine is superior to other sedating antihistamines.
- Alternative first-generation antihistamines, such as chlorphenamine or promethazine, offer a more cost-effective option.
- Alimemazine is unlicensed in the UK for insomnia and this indication is not included within the BNF for children
- The BNF states that “Expert sources advise alimemazine tartrate is used in children from the age of six months to two years for the treatment of urticaria and pruritus, but it is not licensed for this age group”. Alimemazine is contraindicated in children under two due to respiratory depression except on specialist advice.
- Following discontinuation of the Vallergan® brand, over the last two years the cost of alimemazine has increased by more than 1500%.
- Current prices of £112.88 per pack of 28 x 10 mg tablets, £243.51 per 100 ml of 30mg/5ml oral and £179.58 per 100 ml of 7.5mg/5ml oral solution.
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £612,902 (2022/23)
Amiodarone
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- May be suitable in individuals prior and post cardioversion or in specific individuals who also have heart failure or left ventricular impairment.
- Must be initiated by a specialist and only continued for individuals where other treatments cannot be used, have failed or is in line with NICE Guidance CG180.
- Where there is an existing cohort of individuals taking amiodarone it is recommended that these individuals are reviewed to ensure that prescribing remains safe and appropriate, and necessary monitoring is in place.
Background and rationale for recommendation
- Amiodarone is used for treatment of arrhythmias, particularly when other drugs are ineffective or contra-indicated and should be initiated in hospital or under specialist supervision. It is indicated for:
- tachyarrhythmias associated with Wolff-Parkinson-White Syndrome
- atrial flutter and fibrillation when other drugs cannot be used
- and all types of tachyarrhythmias of paroxysmal nature including: supraventricular, nodal, and ventricular tachycardias, ventricular fibrillation; when other drugs cannot be used.
- NICE guidance on atrial fibrillation puts greater emphasis on rate rather than rhythm control and has clarified the place of amiodarone and dronedarone in the treatment pathway.
- It has potentially serious adverse effects and its use requires regular monitoring both clinically and via laboratory testing.
Category:
- Items of limited clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns..
Annual Spend:
- £86,913 (2022/23)
Ascorbic acid
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- Treatment of scurvy.
- Medically diagnosed deficiency, including for those individuals who may have a lifelong or chronic condition or have undergone surgery that results in malabsorption. Continuing need should however be reviewed on a regular basis.
Background and rationale for recommendation
- Ascorbic acid (vitamin C) tablets are indicated for the prevention and treatment of scurvy.
- The Department of Health and Social Care recommends that people should be able to get all the vitamin C they need by eating a varied and balanced diet.
- There is some evidence to indicate a minor benefit in using ascorbic acid in the prevention and treatment of the common cold however routine supplementation cannot be justified.
Category:
- Items of limited clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £489,627 (2022/23)
Buprenorphine patches (7-day transdermal patch – 5 to 20 micrograms)
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- 7-day transdermal patches (5 to 20 micrograms) when prescribed in line with SMC restriction: For use in elderly individuals (over 65 years) for the treatment of non-malignant pain of moderate intensity when an opioid is necessary for obtaining adequate analgesia.
- Individuals undergoing palliative care, where the recommendation to use buprenorphine 7-day transdermal patches in line with the Scottish Palliative Care guideline, has been made by a multidisciplinary team and/or other palliative care specialist.
Background and rationale for recommendation
- There are several brands of buprenorphine transdermal patches. They are available in different strengths, are not all interchangeable.
- 7- day transdermal buprenorphine patches are licensed for treatment of non-malignant pain of moderate intensity when an opioid is necessary for obtaining adequate analgesia.
- Various brands of ‘higher’ strength buprenorphine patches (3-to 4-day patches) are available and licensed for moderate to severe cancer pain and severe pain which does not respond to non-opioid analgesics. These are not included in this guidance.
- To avoid confusion and the potential for dosing errors, buprenorphine transdermal patches should be prescribed by brand name.
- They are not appropriate for individuals with acute pain and those who need rapid dose escalation.
- Butec® patches are restricted by the SMC for use in elderly individuals (over 65 years) for the treatment of non-malignant pain of moderate intensity when an opioid is necessary for obtaining adequate analgesia
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £1,438,242 (2022/23)
Chloral hydrate/Cloral betaine
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- Short term use (less than two weeks) in adults initiated by a specialist for severe insomnia, when insomnia is interfering with daily life and other therapies have failed
- Short term use (less than two weeks) in children and adolescents with a suspected or definite neurodevelopmental disorder, which has been initiated by a specialist for severe insomnia, where the benefits of short-term use outweigh any potential risk.
- Off-label use of chloral hydrate in the management of children and young people with movement disorders initiated and monitored by a specialist.
Background and rationale for recommendation
- Chloral hydrate is indicated for the short-term treatment of severe insomnia which is interfering with normal daily life and where other therapies have failed; as an adjunct to non-pharmacological therapies. It is used for a range of indications including nighttime sedation; management of dystonia and other movement disorders; sedation in critical care; and sedation for painless procedures.
- The BNF classes chloral hydrate/cloral betaine as being less suitable for prescribing in insomnia. It has a narrow therapeutic index and has been associated with individual fatalities.
- Due to the potential for carcinogenic effects, based on animal data, a MHRA drug safety update in October 2021 further restricted the paediatric indication for chloral hydrate/cloral betaine to only children and adolescents with a suspected or definite neurodevelopmental disorder where the benefits of short-term use outweigh any potential risk.
- Treatment should be for the shortest duration possible, should not exceed two weeks and should be under the supervision of a medical specialist.
- Following prolonged treatment, the dose should be slowly tapered down as abrupt discontinuation can lead to delirium.
- Following above MHRA advice, the Neonatal and Paediatric Pharmacist Group (NPPG) in conjunction with the British Academy of Childhood Disability (BACD) and the British Paediatric Neurology Association (BPNA) issued a position statement aimed to help clarify the off-label use of chloral hydrate in the management of children and young people with movement disorders. It states: “When using chloral hydrate for movement disorders, use the lowest effective dose, at the lowest frequency and for the shortest period possible. The need for ongoing use should be regularly assessed and documented”.
Category:
- Items of limited clinical effectiveness, where there is a lack of robust evidence of their effectiveness or significant safety concerns.
Annual Spend:
- £673,632 (2022/23)
Dosulepin
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- None
Background and rationale for recommendation
- Dosulepin is a tricyclic antidepressant and is licensed for the treatment of symptoms of depressive illness especially where sedation is required.
- In December 2007, the MHRA issued safety advice around prescribing of dosulepin, related to the narrow margin between therapeutic doses and potentially fatal doses.
- It is marked as ‘less suitable for prescribing’ in the BNF because relative incidence and severity of side effects is higher than other antidepressants, together with the risk of toxicity, and potential drug interactions.
- Dosulepin should not be initiated in primary care for any indication and existing individuals should be reviewed for suitability for switching to a safer agent. This may require consultation with a specialist.
- Dosulepin should not be stopped abruptly unless serious side effects have occurred.
Category:
- Items of limited clinical effectiveness, where there is a lack of robust evidence of their effectiveness or significant safety concerns.
Annual Spend:
- £611,489 (2022/23)
Dronedarone
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- Should only be prescribed for individuals who meet the SMC restriction criteria: prevention of recurrence of AF in individuals in whom beta-blockers, class 1c drugs or amiodarone are contra-indicated, ineffective or not tolerated and when appropriate monitoring is in place. Treatment should be initiated on specialist advice only.
Background and rationale for recommendation
- Dronedarone is indicated for the maintenance of sinus rhythm after successful cardioversion in adult clinically stable individuals with paroxysmal or persistent atrial fibrillation after alternative treatment options have been considered.
- A MHRA drug safety update in December 2014 concluded that a review due to new evidence of cardiovascular, hepatic and pulmonary risk, found that the benefits of treatment continue to outweigh the risks for the maintenance of sinus rhythm after successful cardioversion in a limited population of individuals with paroxysmal or persistent atrial fibrillation; however, in light of safety concerns dronedarone should only be prescribed after other treatment options have been considered.
- NICE guidance on atrial fibrillation puts greater emphasis on rate rather than rhythm control and has clarified the place of amiodarone and dronedarone in the treatment pathway.
Category:
- Items of limited clinical effectiveness, where there is a lack of robust evidence of their effectiveness or significant safety concerns.
Annual Spend:
- £396,513 (2022/23)
Immediate release (IR) fentanyl
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- Individuals undergoing palliative care, where the recommendation to use immediate-release fentanyl in line with the Scottish Palliative Care guideline has been made by a multidisciplinary team and/or other palliative care specialist.
Background and rationale for recommendation
- Fentanyl is a strong opioid analgesic. It is available in various forms, such as tablets, lozenges, film, and nasal spray, and is licensed for the treatment of breakthrough pain in adults with cancer who are already receiving at least 60mg oral morphine daily or equivalent.
- IR fentanyl products have different absorption and elimination characteristics and are not interchangeable.
- The SMC have advised that Abstral® sublingual tablets, Effentora® buccal tablets, Instanyl® nasal spray and PecFent® nasal spray, should be restricted for use within NHS Scotland for the management of breakthrough pain in adults using opioid therapy for chronic cancer pain, when other short-acting opioids are unsuitable
Category:
- Items that are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £650,482 (2022/23)
Lidocaine plasters
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- Prescribe to individuals in line with SMC guidance, who are who are still experiencing neuropathic pain associated with previous herpes zoster infection (post-herpetic neuralgia, PHN). Its use is restricted to individuals who are intolerant of first-line systemic therapies for post-herpetic neuralgia or where these therapies have been ineffective.
- Individuals undergoing palliative care, where the recommendation to use lidocaine plasters in line with the Scottish Palliative Care guideline has been made by a multidisciplinary team and/or other palliative care specialist.
Background and rationale for recommendation
- Lidocaine plasters can be applied for pain relief and are licensed for symptomatic relief of neuropathic pain associated with previous herpes zoster infection (post-herpetic neuralgia, PHN) in adults.
- lidocaine 5% medicated plaster is accepted by the SMC for restricted use within NHS Scotland for the treatment of neuropathic pain associated with previous herpes zoster infection (post-herpetic neuralgia).
- NICE guidance on chronic pain does not recommend lidocaine plasters for treating neuropathic pain.
Category:
- Items of limited clinical effectiveness, where there is a lack of robust evidence of clinical effectiveness or significant safety concerns.
Annual Spend:
- £13,110,276 (2022/23)
Liothyronine
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- These recommendations do not apply to individuals who have already been reviewed by an NHS consultant endocrinologist.
- New patients with overt hypothyroidism whose symptoms persist on levothyroxine may be prescribed liothyronine after a three-month or longer review by an NHS consultant endocrinologist.
- These recommendations do not apply to individuals with thyroid cancer where liothyronine is used in preparation for radioiodine ablation, iodine scanning, or stimulated thyroglobulin test.
- These recommendations do not apply to individuals where liothyronine is prescribed for the treatment of depression only, in certain settings under the care of a NHS consultant psychiatrist.
Background and rationale for recommendation
- Liothyronine (also known as T3) is used to treat hypothyroidism. It has a similar action to levothyroxine but is more rapidly metabolised and has a more rapid effect. Levothyroxine is the first line treatment for thyroid hormone replacement. It has a long half-life which enables once daily dosing and stable plasma T4 levels. Liothyronine has a shorter half-life and steady state levels cannot be achieved with once daily dosing.
- The majority of people with hypothyroidism can be managed with levothyroxine. However, a small proportion of individuals treated with levothyroxine continue to suffer with symptoms despite adequate biochemical correction.
- T4 monotherapy should be considered first line in the treatment of primary hypothyroidism as it remains the safest and most effective treatment for most individuals.
- The combined use of levothyroxine and liothyronine should only be initiated by an endocrinologist.
- The price of liothyronine has fallen but it is still significantly higher than the price of levothyroxine tablets.
- The British Thyroid Association (BTA) and Society for Endocrinologists 2023 joint consensus statemen states “There is no convincing evidence to support routine use of thyroid extracts, L-T3 monotherapy, compounded thyroid hormones, iodine containing preparations, dietary supplementation and over the counter (OTC) preparations in the management of hypothyroidism”.
- Individuals who have not had a review and are already established on liothyronine as monotherapy or in combination with levothyroxine should have a review by an NHS consultant endocrinologist.
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £1,334,000 (2022/23)
Trimipramine
Recommendation:
- Prescribe only if for an exception or indication named in this guidance.
- Consider deprescribing in individuals currently prescribed this item where appropriate.
- Prescribe only if no other item or intervention is clinically appropriate.
- Prescribe only if no other item or intervention is available.
Exceptions:
- None
Background and rationale for recommendation
- Trimipramine is a tricyclic antidepressant (TCA) with sedative properties. It is licensed for the treatment of depressive illness, especially where sleep disturbance, anxiety or agitation are presenting symptoms.
- Trimipramine is significantly more expensive than other antidepressants e.g. trimipramine 25mg tablets are £200.50 for 28.
- Trimipramine should not be stopped abruptly unless serious side effects have occurred. The dose should preferably be reduced gradually.
Category:
- Items which are clinically effective but more cost-effective products are available, including products that have been subject to excessive price inflation.
Annual Spend:
- £557,653 (2022/23)
4.2 Blood Glucose Monitoring Strips and Insulin Pen Needles
Blood glucose monitoring strips (costing more than £10 for 50 strips)
Recommendation:
- Do not initiate strips that cost over £10 for 50 strips
- Deprescribe or change in individuals currently prescribed this item
- Prescribe only if no other item or intervention is clinically appropriate
- Prescribe only if no other item or intervention is available
Exceptions:
- These recommendations do not apply to blood glucose monitoring strips that cost less than £10 for 50 strips.
Background and rationale for recommendation:
- There are currently over 40 different types of blood glucose testing strips available in the UK. They range in price from £5.45 to £16.40 per 50 strips. Rationalising use ensures that the most cost-effective options are used first line.
- The inclusion of BGM strips in this guideline is not to discourage prescribing it is to encourage health boards and prescribers to consider more cost-effective alternatives and to ensure blood glucose monitoring is only recommended when appropriate.
- Self-monitoring of blood glucose is not routinely recommended for individuals with type 2 diabetes who are controlled with metformin, pioglitazone, SGLT2 inhibitor, gliptin, or GLP-1 mimetic if not prescribed insulin, a sulfonylurea or a repaglinide.
- Self-monitoring of blood glucose is not routinely recommended for individuals with type 2 diabetes who are controlled with diet and exercise.
Category:
- Items which are clinically effective but more cost-effective products are available, including products which have been subject to excessive price inflation.
Annual Spend:
- £3,429,851 (2022/23)
Insulin pen needles (costing more than £5 per 100 needles)
Recommendation:
- Do not initiate strips that cost over £5 for 100 needles
- Deprescribe or change in individuals currently prescribed this item
- Prescribe only if no other item or intervention is clinically appropriate
- Prescribe only if no other item or intervention is available
Exceptions:
- These recommendations do not apply to blood glucose monitoring strips that cost less than £5 for 100 needles.
Background and rationale for recommendation:
- There are a large variety of insulin pen needles available costing from £3.95 to £30.08 for 100.
- The Forum for Injection Technique (FIT) UK considers the 4mm needle to be the safest pen needle for adults and children regardless of age, gender, and Body Mass Index (BMI).
- Using needles of a shorter length helps to prevent intramuscular injection of insulin. For individuals currently using longer pen needle lengths (8mm, 12mm), it is advisable to change to a shorter needle length (6mm or less) but only after discussion with a healthcare professional, to ensure they receive advice on the correct injection technique.
Category:
- Items which are clinically effective but more cost-effective products are available, including products which have been subject to excessive price inflation.
Annual Spend:
- £600,559 (2022/23)
4.3 Antimicrobial Stewardship
The aim of this paper is to provide recommendations on the effective prescribing of antimicrobials as part of the national value-based health and care work (VBH&C). The guidance on the use of antimicrobials complements the work of the Scottish Antimicrobial Prescribing Group (SAPG), which promotes the safe and effective use of antibiotics across hospital and community settings to improve antibiotic stewardship and tackle the risks of antimicrobial resistance.
The work of SAPG and the VBH&C group is aligned to Realistic Medicine and the Climate Emergency and Sustainability agenda in Scotland. Appropriate use of antibiotics helps to reduce unwarranted variation in prescribing and maximise value of healthcare, achieving better outcomes for the individual and leading to the creation of a more sustainable health care system.
Background
Antibiotic resistance is a major global public health issue and a threat to the future of healthcare as described in this WHO factsheet.
Residues of medicines, including antibiotics, can be found in water, soil and sludge and can accumulate in living organisms. The ecotoxicological effects have been linked with assisting the spread of antimicrobial resistance and lasting effects on biodiversity.
Choice to prescribe antibiotic treatment
A key antimicrobial stewardship approach to optimising antibiotic use in primary care is minimising their use for symptoms such as coughs, colds, sore throats, and earache in otherwise fit and healthy people. Antibiotics are not required for viral infections. Self-care and symptom relief should be recommended, for example rest, adequate pain relief and hydration as well as reassurance regarding expected duration of symptoms and actions to take if symptoms worsen or persist. This avoids the risk of increasing exposure to unnecessary antibiotics, adding to issues with antibiotic resistance and avoiding the risk of side effects or medicine interactions. The top ten antibiotics prescribed in NHS Scotland primary care in 2023 are highlighted below.
Table 1: Top 10 antibiotics prescribed in primary care - Total items prescribed for year 2023
- Amoxicillin - 1,089,965
- Doxycycline - 512,849
- Trimethoprim - 437,227
- Flucloxacillin - 436,672
- Phenoxymethylpenicillin - 385,175
- Nitrofurantoin - 310,177
- Clarithromycin - 191,008
- Metronidazole - 175,393
- Co-Amoxiclav - 146,779
- Lymecycline - 111,891
Duration of antibiotic treatment
Antimicrobial resistance is a natural consequence of using antibiotics, but overuse and inappropriate use can increase the rate of development of resistance.
An important element of antibiotic stewardship is making sure that when antibiotics are clinically indicated, they are not given for any longer than required, and that advice to the patient is to ‘complete the full course’, as partial treatment can increase the risk of antimicrobial resistance.
Five-day treatment courses for respiratory tract infections
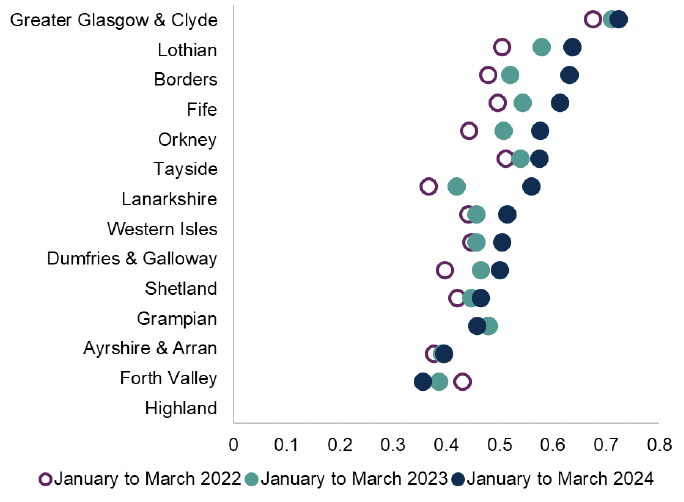
Respiratory infections are the main infection related consultation in NHS Scotland and the most common reason for antibiotics prescribed in the community. Where prescribing is indicated, evidence exists that initial five-day treatment courses are recommended for community acquired and hospital acquired pneumonia. In 2022, 64.7% of amoxicillin 500mg courses, across all indications, were for five days duration, compared to 58.1% in 2021. This may suggest improving compliance with antibiotic prescribing policies, however there is room for further optimisation. In Scotland the most common antibiotics prescribed in the treatment of respiratory conditions are amoxicillin, doxycycline and phenoxymethylpenicillin and to a lesser extent clarithromycin and co-amoxiclav which are used in lower volumes.
Chart 1: Five day courses of amoxicillin, doxycycline and clarithromycin as a proportion of all amoxicillin, doxycycline and clarithromycin items
Three-day treatment courses for uncomplicated lower urinary tract infections in women.
Chart 2: Number of 3-day courses of acute UTI antibiotics prescribed to women as a proportion of all acute UTI antibiotic courses prescribed to women
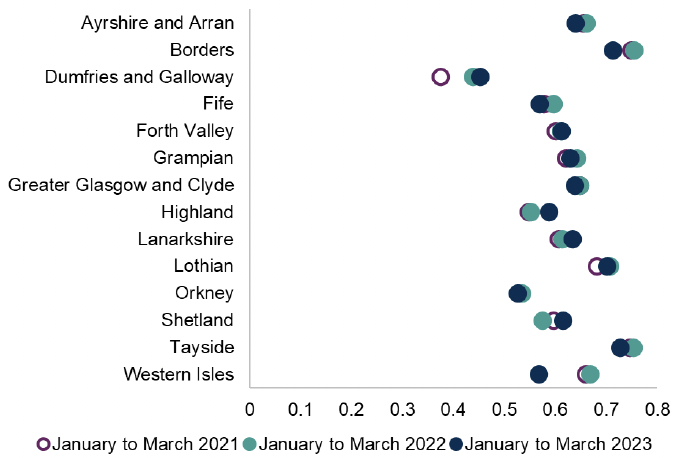
Trimethoprim and Nitrofurantoin are the most commonly used antibiotics for uncomplicated lower urinary tract infection in NHS Scotland. When antibiotics are indicated the recommended treatment course duration is three days in women.
The National Therapeutic Indicator above (figure 3) shows the prescribing of three-day treatment courses continues to show variation between health boards across NHS Scotland. Reducing exposure to unnecessary antibiotic use or avoiding unnecessary prolonged treatment courses can slow the development of antibiotic resistance.
Antibiotic prescribing and consultation type
There are a variety of types of consultation used in practice including face to face consultations with an increased use of remote consultations, either telephone, video (NHS Near Me) or e-consultations since the COVID-19 pandemic. Concern has been raised that some forms of remote consultation (e.g. telephone) may increase the likelihood of antimicrobial prescribing in adults.
Use of intravenous antibiotics
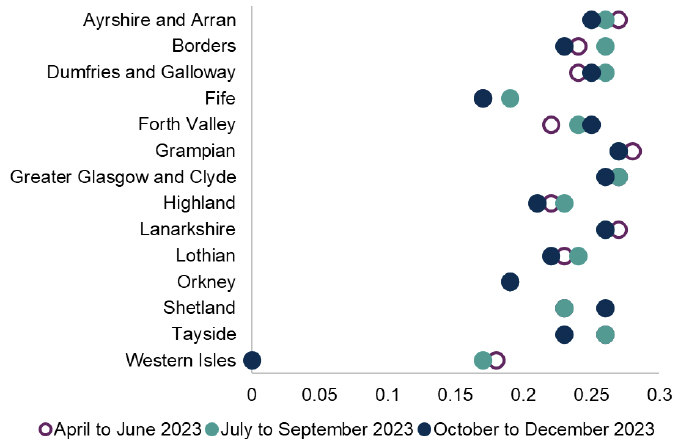
In Scotland in 2022 antibiotics given intravenously accounted for around 30% of total antibiotic use in acute hospitals. The work on clinical review led by SAPG has focussed on patients started on IV antibiotics and aims for a documented management plan including rationalisation within 72 hours of starting treatment. This aims to reduce unnecessary continuation of antibiotics, ensure personalised treatment and ensure appropriate IV to oral switch. This brings associated benefits for patients of reduced risk of device-related infections and potential for earlier discharge from hospital. Use of oral antibiotics also reduces the use of plastics, e.g. giving sets, when compared to IV antibiotics.
The National therapeutic indicator below shows injectable antibiotics as a proportion of all antibiotics prescribed in secondary care by NHS board across NHS Scotland.
Chart 3: Injectable antibiotic defined daily doses (DDDs) as a proportion of all antibiotic DDDs
Use of solid oral dosage forms of antibiotics
Life cycle assessments (LCA) of various dosage forms illustrates that solid oral dosage forms have a reduced climate change impact compared to liquids. One study illustrates that paracetamol syrup production has a greater contribution to climate change and environmental impact compared to tablets. Solid oral dosage forms are often safer (licensed), more cost effective and more acceptable to patients and carers. Tablet distribution is simpler, often they have a longer shelf life and create less packaging waste. Pill swallowing is a skill that can be taught to children above the age of four and adults if needed.
Safe disposal of pharmaceuticals
Reducing medicine waste can be done through reducing the inappropriate use of antibiotics, ensuring the optimum treatment course is prescribed and that any unused medicines, including antibiotics, should be returned to the community pharmacy for safe disposal by incineration. No medication should be flushed down the toilet or put into domestic bins to minimise ecotoxicological effects. There are national posters (produced by Scottish Water’s Nature Calls campaign and Scottish Government’s Antibiotic Amnesty) which encourage safe disposal of any medicines prescribed.
Recommendations
- Avoid prescriptions for antibiotics in self-limiting or viral infections to reduce the risk of antibiotic resistance. Promote self-management for these situations: e.g., rest, symptomatic relief and hydration. Encourage people to become antibiotic guardians.
- Ensure that antibiotic courses are prescribed for the appropriate duration. (e.g., three days for uncomplicated UTI’s in women and five days for community acquired and hospital acquired pneumonia)
- Reinforce advice that antibiotics are taken as directed to ensure effectiveness (Take at regular intervals, do not skip doses and check that the full course is taken). Advise that patients do not save antibiotics for later or share unused antibiotics with family or friends.
- Ensure a management plan is documented within 72 hours when commencing IV antibiotics, including consideration of continuation and rationalisation of treatment with the potential for stopping, or switching IV to oral therapy.
- Prescribe oral solid dosage forms where possible, minimising use of liquid preparations or IV to where they are necessary and appropriate.
- Offer resources and advice to patients and families on learning how to swallow pills.
- Encourage that any unused medicine is returned to community pharmacy for safe disposal.
- Signpost and encourage healthcare staff to update education in antibiotic management using SAPG and NES materials on TURAS.
Appendix 1 – Potential unintended consequences from implementation of this guidance
Unintended consequence: Interactions with specialists in secondary care and consequent costs.
Response: This will need monitoring but is not inevitable. For some products, joint local guidance with secondary care providers may be appropriate.
Unintended consequence: Use of appointments in primary care, for example pain management reviews.
Response: There could initially be an increased use of appointments in primary care to improve appropriateness of prescribing, however this is not expected to be sustained.
Unintended consequence: Some substitute treatments may not be clinically identical, such as side-effect profile
Response: Prescribers should make shared decisions with patients, and appropriate resources should be provided to facilitate this e.g. Patient information leaflets
Unintended consequence: Substitute treatments could, in some cases, be prescribed with cost consequences
Response: This is an opportunity to review medication, and if appropriate to deprescribe, although alternatives may need to be considered.
Unintended consequence: Individual prescribers’ decision making
Response: Prescribers must recognise and work within the limits of the competence, as recommended by the General Medical Council (GMC) and other professional regulators/bodies. The proposed guidance does not remove the clinical discretion of the prescriber in deciding what is an accordance with their professional duties.
Unintended consequence: People currently on treatment stopping or altering their treatments without appropriate advice
Response: Prescribers should endeavour to explain the rationale for any proposed changes in treatments and come to a shared decision prior to any changes.
Unintended consequence: Complaints about general practice and associated administration time
Response: To support communication of the changes proposed in the guidance, educational aids will be made available.
Unintended consequence: Effect on medicines supply
Response: By proposing guidance on individual items, there is potential for substitute items to see increased demand. NHS Scotland will work with Department of Health colleagues to ensure that pharmaceutical companies are aware of the proposed guidance and potential need for increased supply of alternative products. Proposed changes will also be communicated to community pharmacies.
Unintended consequence: In some instances substitute therapies may carry a higher risk of dependence and withdrawal.
Response: Effective National and local strategies are required to support the delivery of alternative treatment options, particularly in chronic pain management (e.g. lidocaine). This is important to support safe deprescribing and prevent the increased prescribing of medicines with the potential for harm, dependence or withdrawal.
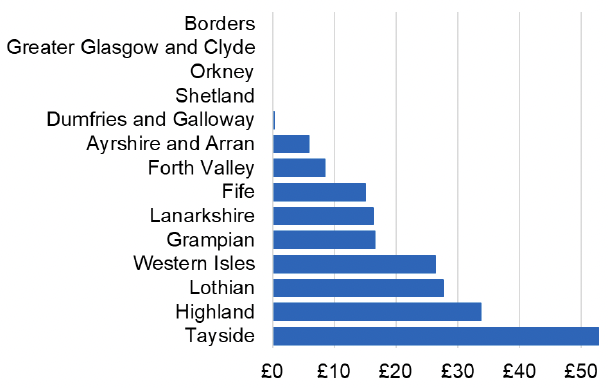
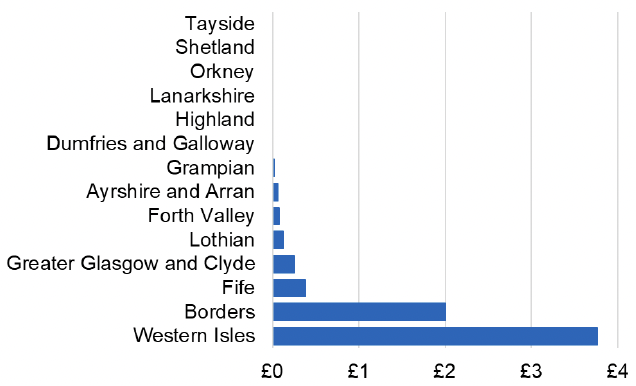
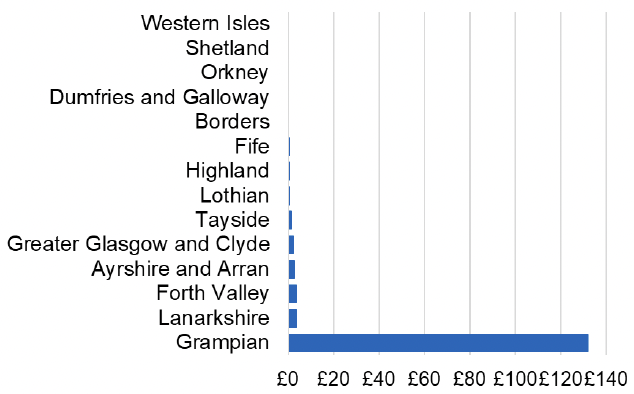
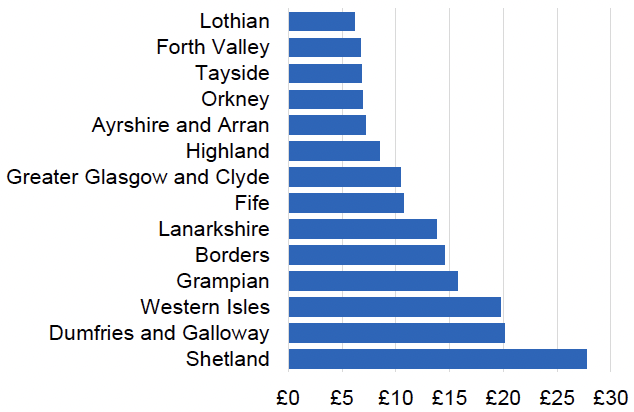
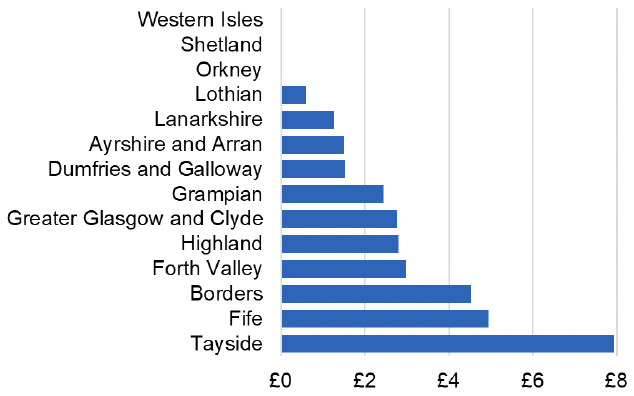
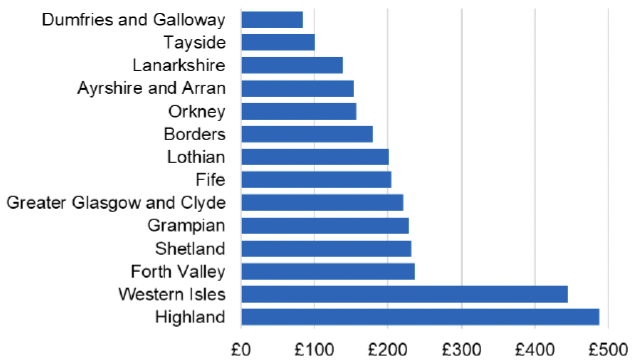
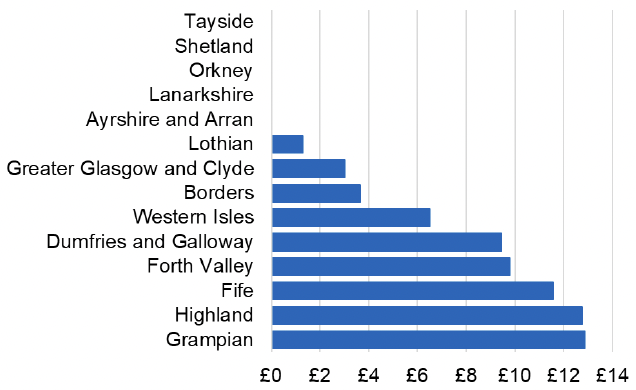
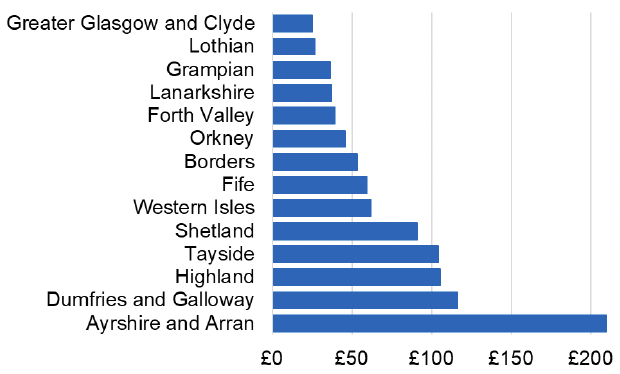
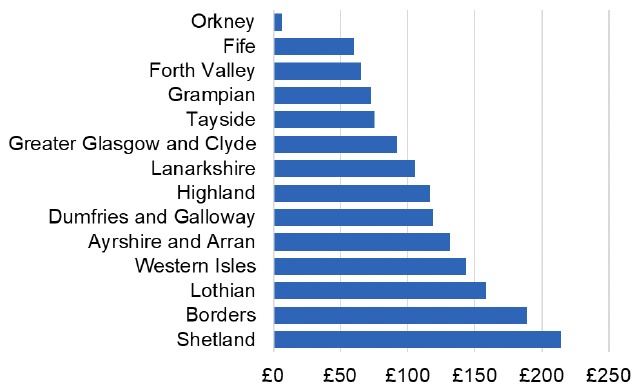
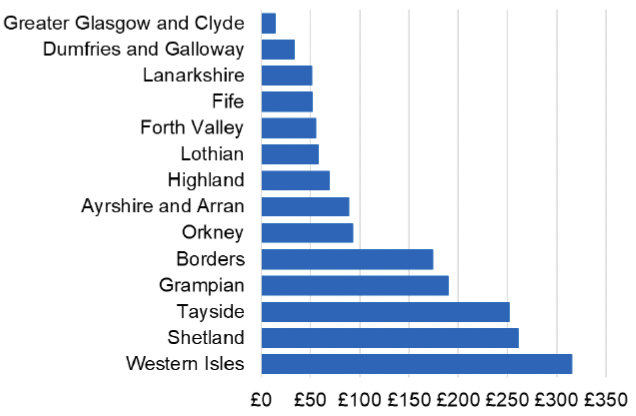
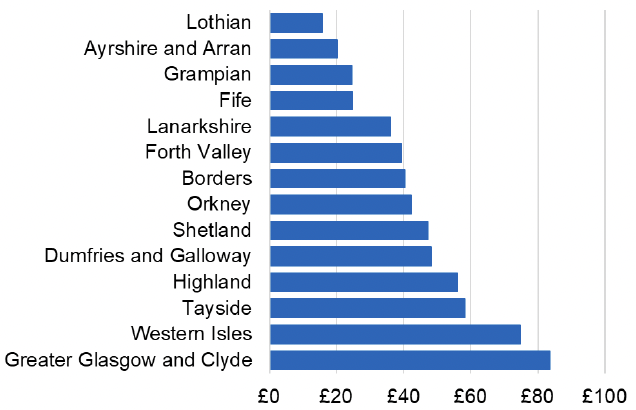
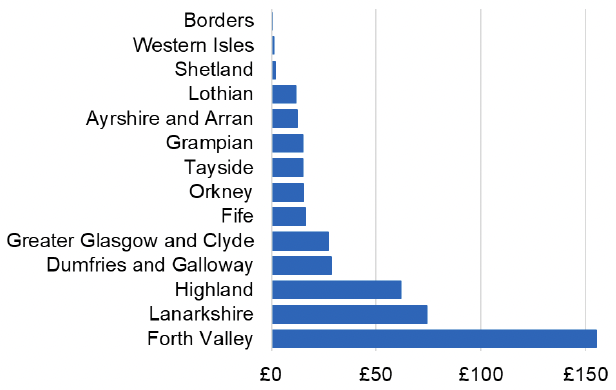
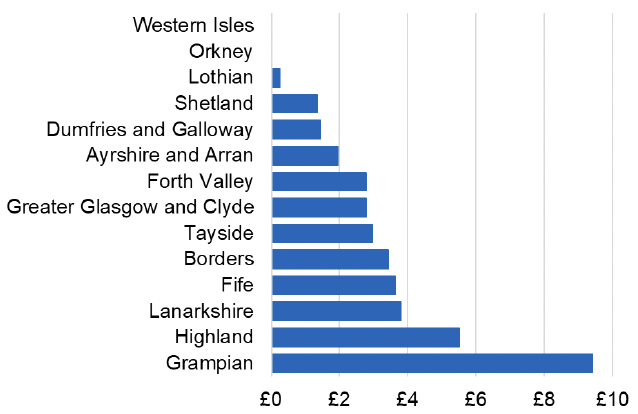
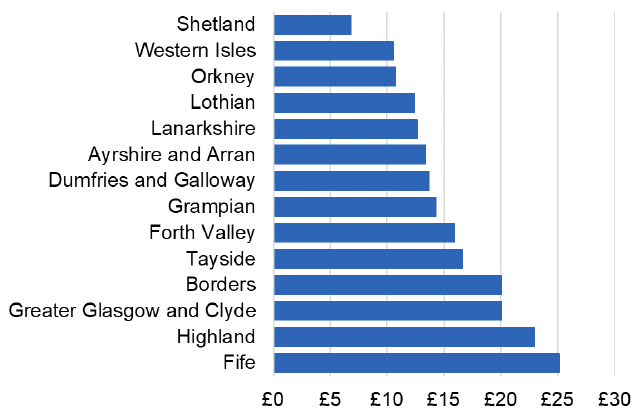
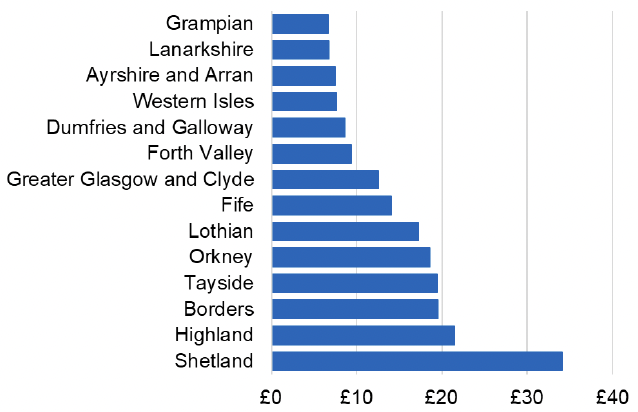
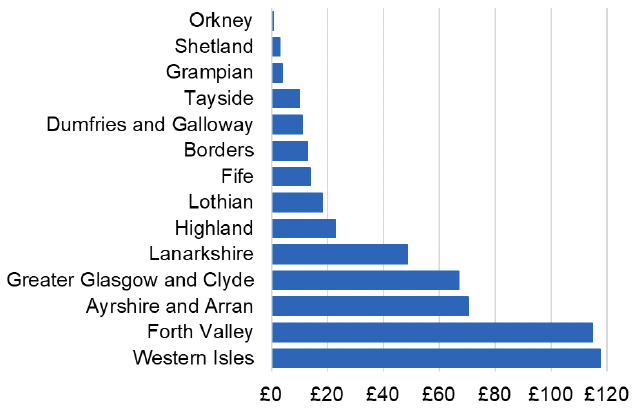
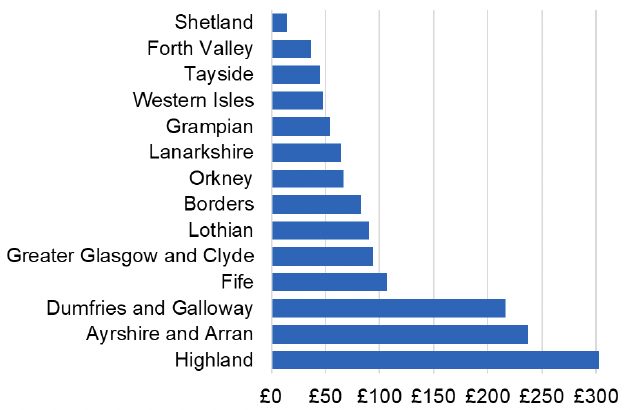
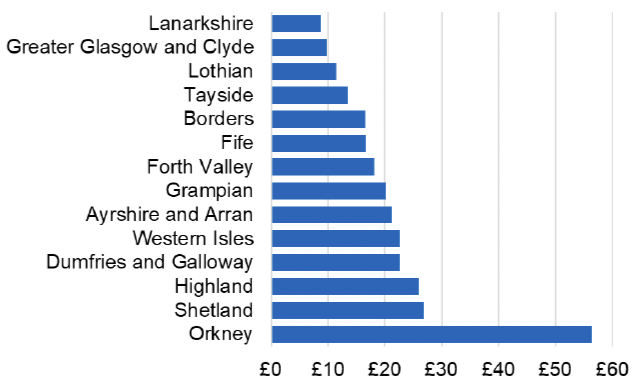
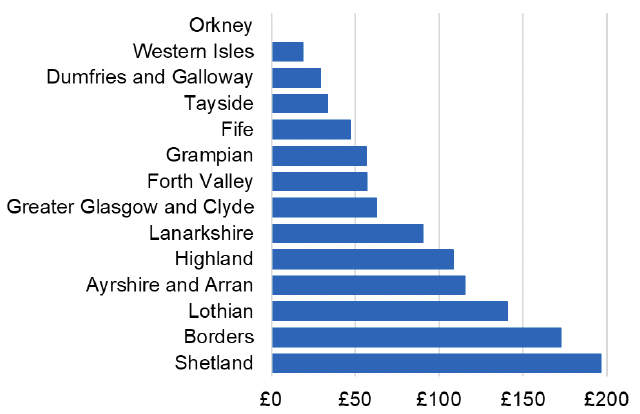
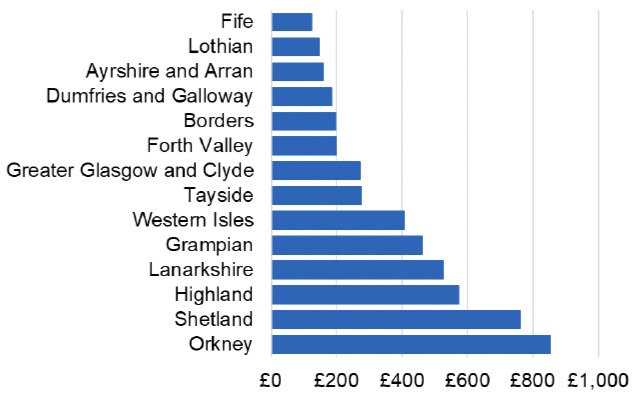
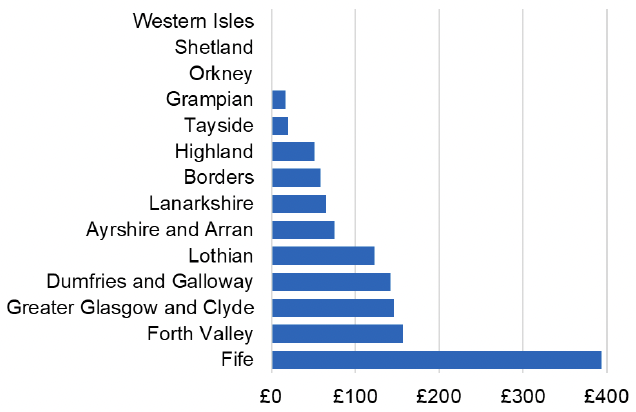
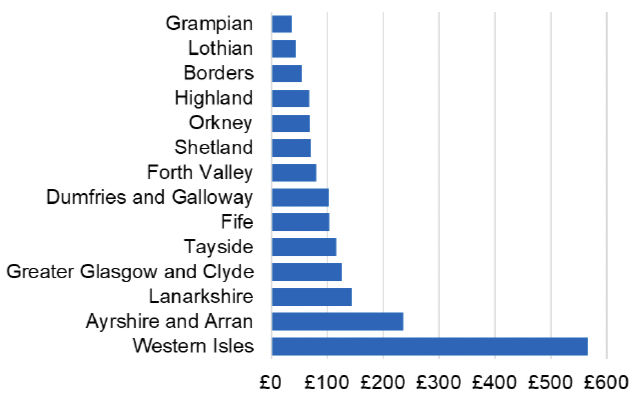
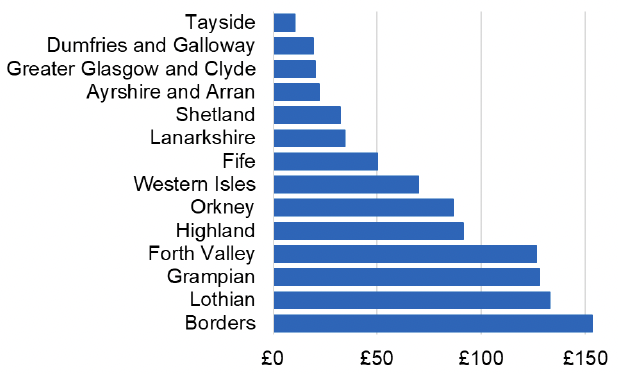
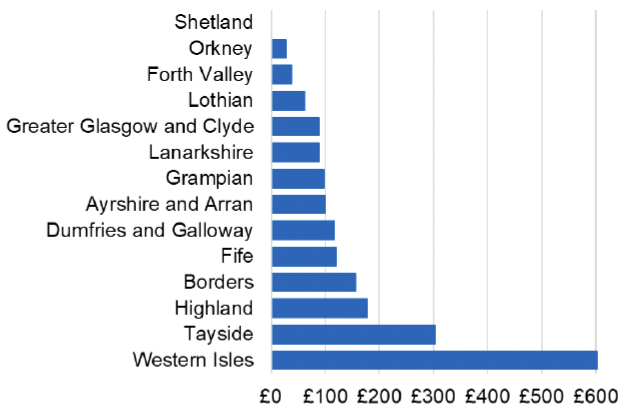
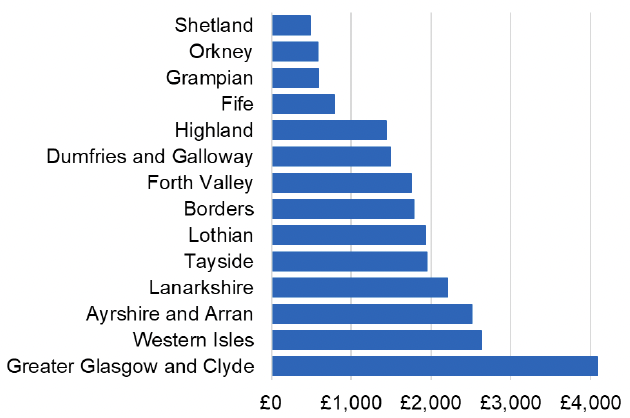
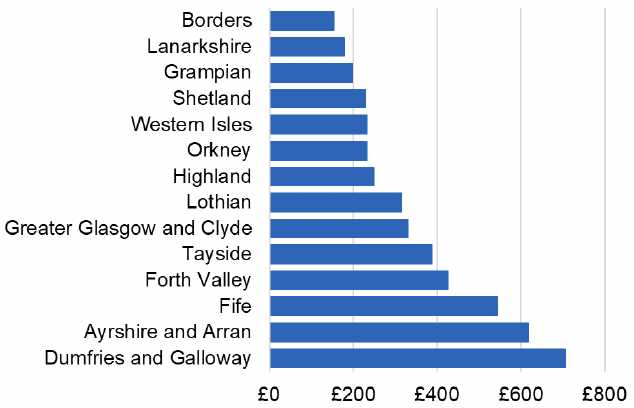
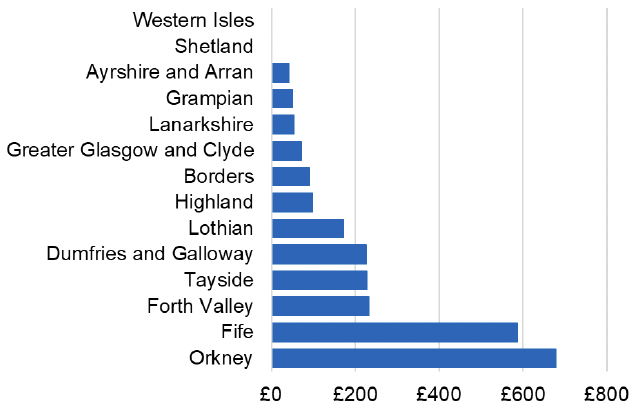
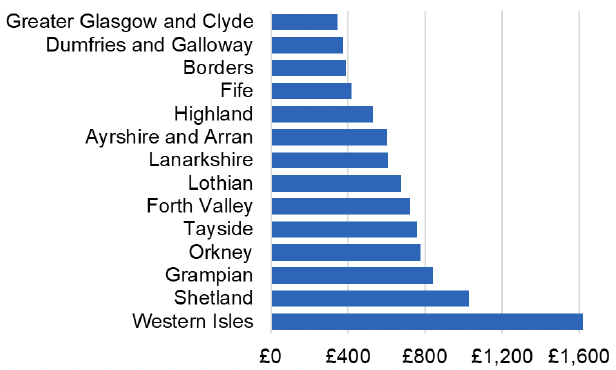
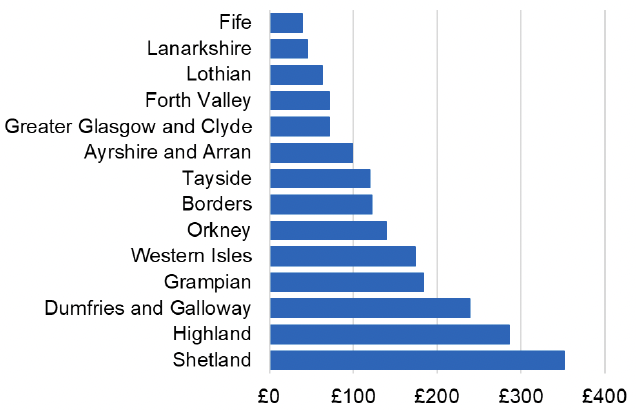
Appendix 2 – Cost per 1,000 Individuals in 2022/23
These charts show the cost per 1,000 individuals (list size) in each NHS Scotland health board. The differences in cost are generally due to higher or lower prescribing rates between boards. For some items where a standard pricing does not apply, variation may also be due to local formulary choices resulting in higher value items being prescribed, for example bath and shower emollients or blood glucose monitoring strips.
Contact
Email: EPandT@gov.scot
There is a problem
Thanks for your feedback