Care Home Data Review - Full Report
The Care Home Data Review (CHDR) is a collaboration between Scottish Government, Public Health Scotland and Care Inspectorate, with the aim of improving the care home data landscape. This report details the feedback to the review and presents recommendations for data improvements.
Annex 2: Flow Chart of Ethical Review Routes for Adult Social Care Data in Scotland
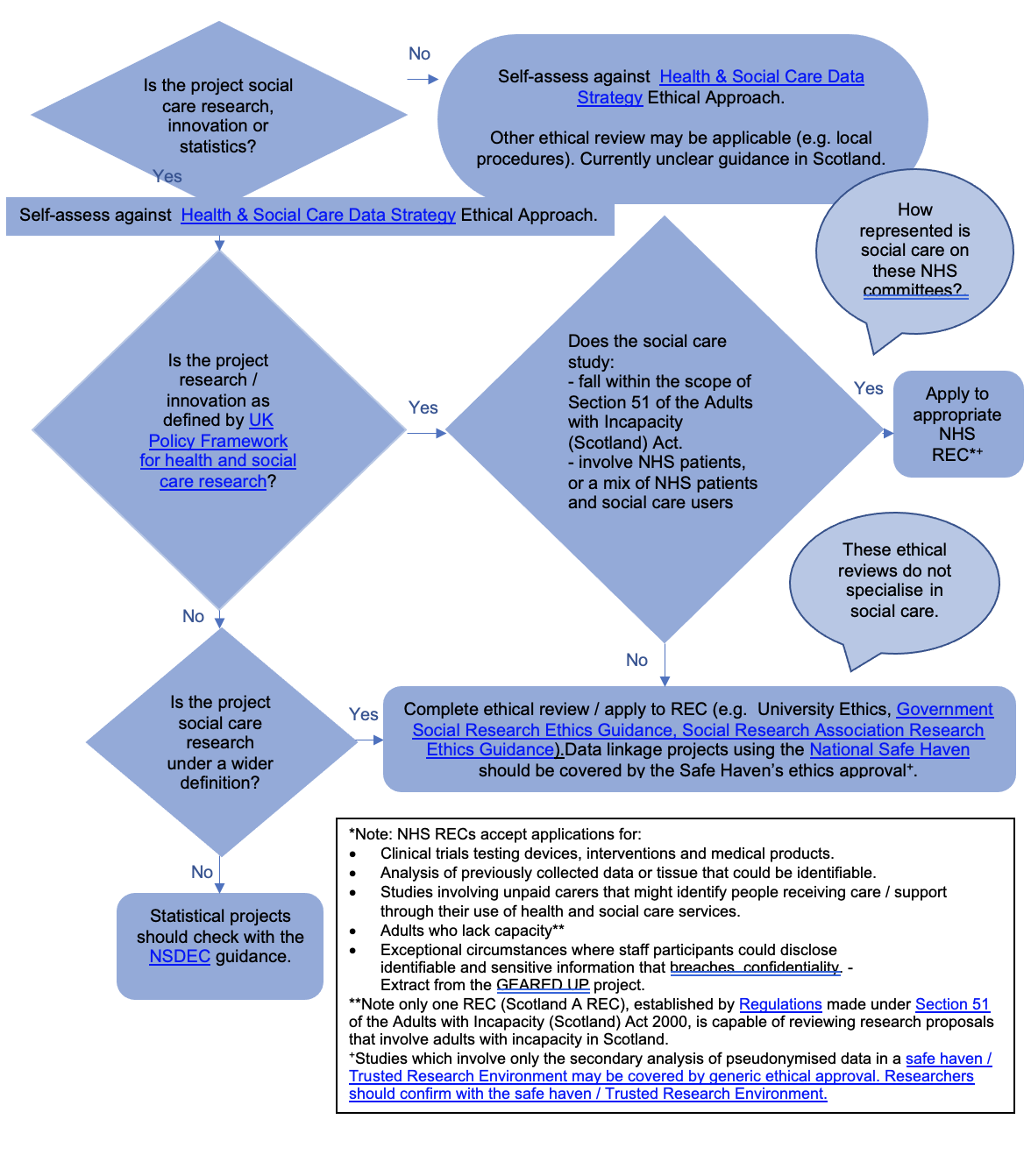
Step 1: Is the project social care research, innovation or statistics?
Yes: Self-assess against Health & Social Care Data Strategy Ethical Approach. Proceed to Step 2.
No: Self-assess against Health & Social Care Data Strategy Ethical Approach. Other ethical review may be applicable (e.g. local procedures). Currently unclear guidance in Scotland.
Step 2: Is the project research / innovation as defined by UK Policy Framework for health and social care research?
Yes: Go to Step 3
No: Go to Step 4
Step 3
Does the social care study:
- fall within the scope of Section 51 of the Adults with Incapacity (Scotland) Act.
- involve NHS patients, or a mix of NHS patients and social care users
Yes: Apply to appropriate NHS REC1,2
Note: It is unclear how represented social care on these NHS committees
No: Complete ethical review / apply to REC (e.g. University Ethics, Government Social Research Ethics Guidance, Social Research Association Research Ethics Guidance ). Data linkage projects using the National Safe Haven should be covered by the Safe Haven’s ethics approval2.
Note – these ethical reviews do not specialise in social care.
Step 4
Is the project social care research under a wider definition?
Yes: Complete ethical review / apply to REC (e.g. University Ethics, Government Social Research Ethics Guidance, Social Research Association Research Ethics Guidance). Data linkage projects using the National Safe Haven should be covered by the Safe Haven’s ethics approval2.
Note – these ethical reviews do not specialise in social care.
No: Statistical projects should check with the NSDEC guidance.
Notes:
1NHS RECs accept applications for:
- Clinical trials testing devices, interventions and medical products.
- Analysis of previously collected data or tissue that could be identifiable.
- Studies involving unpaid carers that might identify people receiving care / support through their use of health and social care services.
- Adults who lack capacity3
- Exceptional circumstances where staff participants could disclose identifiable and sensitive information that breaches confidentiality. Extract from the GEARED UP project.
2 Studies which involve only the secondary analysis of pseudonymised data in a safe haven / Trusted Research Environment may be covered by generic ethical approval. Researchers should confirm with the safe haven / Trusted Research Environment.
3 Note only one REC (Scotland A REC), established by Regulations made under Section 51 of the Adults with Incapacity (Scotland) Act 2000, is capable of reviewing research proposals that involve adults with incapacity in Scotland.
Contact
Email: SWStat@gov.scot
There is a problem
Thanks for your feedback