Conservation of Atlantic salmon - gene banking: principles and considerations
This report examines the procedures, considerations, risks and opportunities of gene banking for Atlantic salmon conservation and restoration in Scotland. Presented is a brief overview of live gene banking and a detailed focus on cryobanking to preserve gametic material through freezing and storage.
Gene banking examples
Live gene banking
Bay of Fundy
The largest live gene banking programme is that run in the Canadian Inner Bay of Fundy (Fisheries and Oceans Canada 2018, Jones et al. 2020). Historically, the various rivers feeding into the Canadian Inner Bay of Fundy consisted of tens of thousands of adult salmon returns. However, returns began to decline in the late 1980s due mainly to poor marine feeding conditions. Population size reductions continued all through the 1990s, and in the year 1999, less than an estimated 250 adults were believed to have returned to the approximately 50 rivers of the inner bay.
To prevent the imminent extirpation of this phenotypically and genetically distinct group of salmon, some of the last remaining juveniles were captured between 1998 and 2001, and transferred to biodiversity facilities in the Maritimes Region of Fisheries and Oceans Canada, for captive breeding and rearing. This Live Gene Banking (LGB) program, and associated juvenile and adult supplementation efforts, has been in operation for over 20 years. However, due to the marine conditions still being very poor, and despite the millions of dollars spent on the programme, there has been little evidence of progress towards the reestablishment of self-sustaining populations (Fisheries and Oceans Canada 2018). Every year, the program releases tens of thousands of fish back to their rivers of origin, however, marine survival has been estimated between 0.05% and 0.62%, with Fisheries and Oceans Canada estimating just 37 adults returned in 2013 and 205 in 2015 (Fisheries and Oceans Canada 2018, Jones et al. 2020).
Thus, the programme can be seen to be not achieving its aim of re-establishing the wild populations of the Bay of Fundy rivers. However, and very importantly, it has ensured that the populations within the bay have not gone extinct (Fisheries and Oceans Canada 2019).
Mixed gene banking
Norwegian Gene Bank
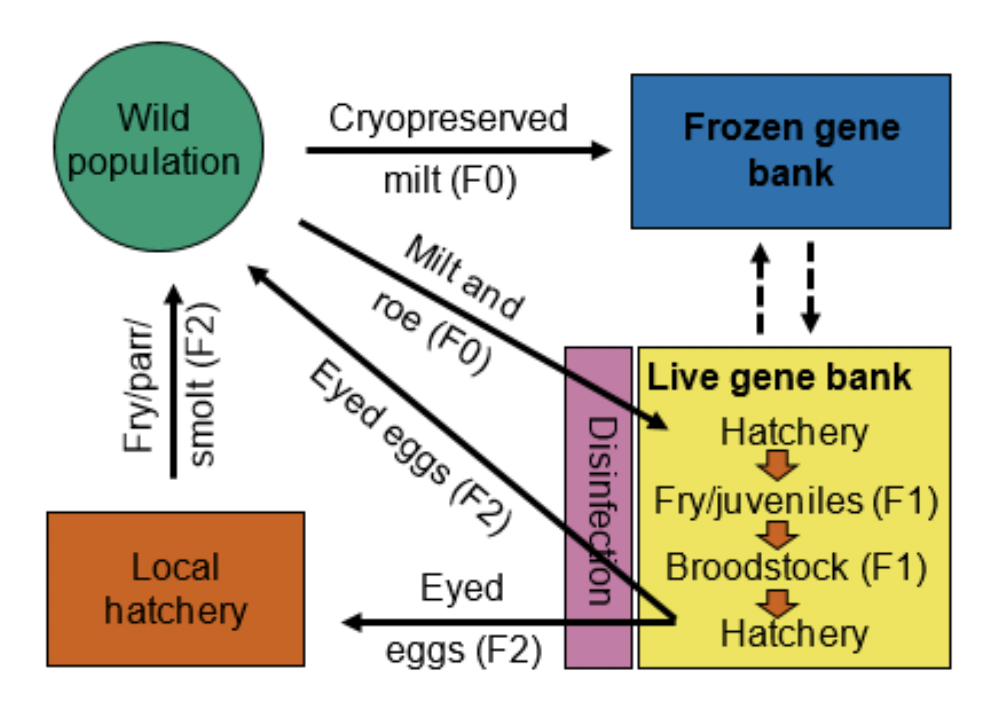
Perhaps the most successful large gene banking programme that incorporates both live and cryobanking is that of the Norwegian Gene Bank (NGB) (Bøe et al. 2021). The NGB was established in 1985 and utilises live gene banks (LGBs) as a temporary living reservoir of genetic material for the reestablishment of living stocks threatened by extirpation. Between the establishment of the first LGB station in 1989 and up to 2021, 68 Atlantic salmon populations have been, or are currently, a part of the LGB programme, 32 of which have successfully been re-established after the eradication of the G. salaris ectoparasite. Furthermore, in an effort to preserve genetic material across the species' natural distribution in Norway, in 2021, sperm from a total of 174 Atlantic salmon populations has been collected and preserved in a cryobank. Although Atlantic salmon was initially the only salmonid included in the gene bank programme, populations from 33 anadromous brown trout (Salmo trutta) and 2 populations of anadromous Arctic charr (Salvelinus alpinus) have now also been incorporated into the programme. The interplay of live and frozen material is outlined in Figure 3.
Figure 3. Flow chart depicting the strategy of the Norwegian national salmonid gene bank. Milt and roe is collected from founder individuals (F0) in the wild and transferred to a hatchery in a live gene bank station. The F0 progeny become the F1 generation broodstock held at the live gene bank station. F1 broodstock produce eyed eggs and/or juveniles released in the wild for population restoration purposes or an F2 generation broodstock at the gene bank when necessary. The gene bank strategy is based on an “eggs in–eggs out” strategy, where disinfected eggs are the only life-stage transferred between the live gene bank and the wild. In cases where juveniles are released to the wild, a local hatchery is used to rear the disinfected eggs. The frozen gene bank contains cryopreserved sperm from wild F0 fish and from broodstock males from the live gene banks. These samples are sometimes used for the production of new broodstock families, or for the production of eggs or juveniles to be released in the wild (taken from Bøe et al. 2021).
Mediterranean trout
An example of cryobanking of particular relevance to small threatened populations of salmonids is that of the LIFE Nat.Sal.Mo project which focused on small populations of endangered Mediterranean brown trout (Di Iorio et al. 2023). In Italy, the populations of native Mediterranean brown trout are in constant and rapid decline with associated dangers of loss of intraspecific biodiversity. The decrease of these native populations is the result of a series of anthropogenic activities, such as river pollution, habitat deterioration, water withdrawals, largely unregulated fishing activities, alien species and hybridisation with alien trout species. Together with habitat conservation, the project included cryopreservation of sperm with a view to preserving the genetic profile of the native populations. Freezing protocols were developed, storage options identified, and opportunities for the restoration of genetic integrity in native populations determined (see Figure 1 above). Frozen semen of native males was then used in appropriate fertilization schemes allowing an increase of the genetic variability in the offspring. During the project timeframe approximately 1,700 semen doses, from 150 native breeders were stored inside the cryobank representing a very important “tank” of genetic variability, as it includes a large number of native donors sampled across a number of spawning seasons. The semen cryobank within the project thus assured the maximum genetic variability during the artificial supportive breeding (Di Iorio et al. 2023).
Contact
Email: John.Gilbey@gov.scot
There is a problem
Thanks for your feedback