Conservation of Atlantic salmon - gene banking: principles and considerations
This report examines the procedures, considerations, risks and opportunities of gene banking for Atlantic salmon conservation and restoration in Scotland. Presented is a brief overview of live gene banking and a detailed focus on cryobanking to preserve gametic material through freezing and storage.
Choice of material to cryopreserve
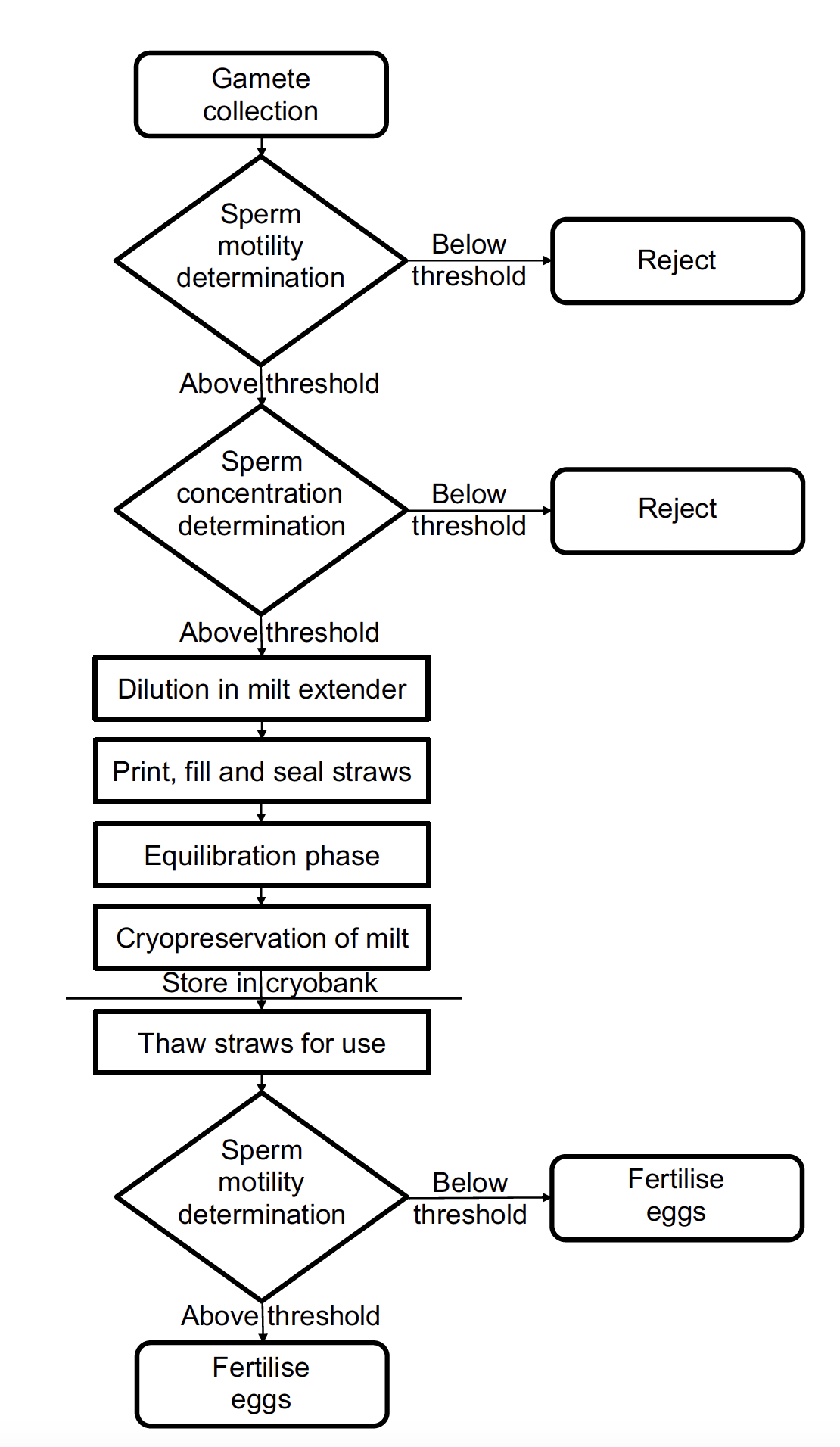
Cryopreserving gametes has been shown to be an effective strategy in the conservation of many fish species (Cabrita et al. 2010, Goswami et al. 2016, Asturiano et al. 2017, Martínez-Páramo et al. 2017, Thipramalai Thangappan et al. 2023), including salmonids (Robles et al. 2003, Judycka et al. 2019, Bøe et al. 2021, Di Iorio et al. 2023). Studies have been carried out using fish oocytes (eggs) and embryos, however, cryopreservation of these has proved difficult due low membrane permeability in the eggs, the large yolk mass of the oocyte, and the presence of compartments in early developing embryos. These factors result in ice crystal formation during the freezing process. In addition, the oocytes and embryos are prone to chilling injuries unrelated to ice crystal damage (Harvey et al. 1983, Kimmel and Law 1985, Zhang and Rawson 1995, Hagedorn et al. 1997, Hagedorn et al. 1998, Zhang and Rawson 1998, Chao and Liao 2001, Zhang et al. 2003, Martínez-Páramo et al. 2017, Diwan et al. 2020). As such, the routine use of oocytes or embryos is not yet feasible (Dunham 2023). Accordingly, sperm cells are the main type of cell used for cryobanking of aquatic species, including salmonids, due to their small size and relatively high resistance to chilling (Sarvi et al. 2006, Martínez-Páramo et al. 2009, Nynca et al. 2015, Martínez-Páramo et al. 2017, Di Iorio et al. 2023, Dunham 2023). There are a number of successful protocols for cryopreservation of sperm in salmonids (e.g. Martínez-Páramo et al. 2009, Dziewulska et al. 2011, Di Iorio et al. 2023). Sperm cryopreserved in this way can be stored almost indefinitely without substantial effect on cell viability or quality (Stoss and Refstie 1983, Bøe et al. 2021)
Cryopreservation of sperm
Cryopreservation of sperm will conserve both the X and Y sex determining chromosomes. As such, all nuclear genomic material will be captured in the cryobanking programme. However, mitochondrial DNA and cellular material found in eggs will not be preserved. Thus, in the absence of native females for reestablishment of the population, any adaptive functions associated with this material will be lost. However, as most (but not all) of the adaptive diversity within the genome is associated with the nuclear DNA (Consuegra et al. 2015), this may be an acceptable outcome. Reestablishment of the population through the use of cryopreserved sperm will, of course, require females to supply eggs for fertilisation. If there are native females available (from the wild or a live gene bank/hatchery) then these can be used in a well-defined crossing programme. In the absence of such females, non-native fish would need to be used. In this case, repeated back-crossings across generations using cryopreserved sperm and marked returning adult females, and/or females retained in a hatchery, allow non-native nuclear genetic material to be removed from the population over time. If using hatchery retained fish, however, care must be taken to minimise any domestication selection due to the retention in the hatchery.
Sperm Collection
The purpose of a cryobanking programme is to sample and retain, as much as is feasible, the existing genetic diversity of a population at the time of sampling (Greene et al. 2014). This may not prove a simple task, due to the complexity of salmon populations in space, time and life-history traits. In most cases, a sophisticated sampling plan should be drawn up in advance, with a rational and statistically valid rationale for sampling choices. Where it is decided that there is not time to undertake such work, another option may be to sample all material possible, and indeed, this would still meet the requirement to capture the existing (remaining) genetic diversity of a population at the time of sampling.
Native fish
It is of paramount importance that only fish from the native population be included in the sampling programme for that stock. Rivers and, indeed, tributaries are divided into a hierarchy of different populations, each adapted to its natal location (Garcia de Leaniz et al. 2007, Cauwelier et al. 2018b, Gilbey et al. 2018). Mixing of stocks will disrupt this local adaptation, resulting in homogenisation of population structure (Vasemagi et al. 2005, Williamson and May 2005, Östergren et al. 2021) and associated loss of genetic local adaptation (McGinnity et al. 2009, Christie et al. 2012). Further, the ability of a species to adapt to environmental changes is dependent on the genetic variability not only within, but also across populations, the so-called ‘Portfolio Effect’ (Figge 2004, Schindler et al. 2015) and, thus, homogenisation of populations will reduce population and species resilience.
Mechanisms are available to ensure only native broodstock are selected, e.g.:
- Tagging smolts and only using tagged adult returners
- Genetically screening fish and using mixture analysis to identify natives
- Collecting milt from precocious parr within the system
Farmed escapes
In areas where aquaculture escapes are a potential issue, it is vital to ensure screening is carried out to prevent incorporation of farmed escapees into any banking programme. Interbreeding between escaped farmed salmon and wild conspecifics and the resulting introgression of genetic material from farm stocks into the wild, brings risks to the diversity, genetic integrity, fitness and viability of wild salmon populations (Naylor et al. 2005, Glover et al. 2020). Genetic screening (pre or post gamete collection) should be used in areas where these risks occur (Gilbey et al. 2021).
Life history representation
The Atlantic salmon has a number of distinct and, to a degree, genetically influenced life history types. These include maturation as parr in fresh water in some males, smolt age, return timing, and sea age (Ayllon et al. 2015, Cauwelier et al. 2018a, Bangura et al. 2022, Ahi et al. 2023, Åsheim et al. 2023, Kess et al. 2024). In order to retain as much as is possible of the existing genetic diversity in the populations being conserved, an appropriate sampling scheme should be employed that captures these various life history genotypes/phenotypes. Further, it is, again, of paramount importance that, when performing fertilisation of eggs at a later stage, that a well-designed crossing scheme is employed, such that the differing life history types are crossed in a way most matching the natural situation.
Replenishment
Cryobanking allows the preservation of genetic material across space and time. Thus, over time, samples from different populations can be replenished so that a time series of samples are available for each population. This provides a comprehensive repository of the genetic material in the wild populations and the differing samples can be used in future crossing schemes to ensure the maximum genetic diversity is both maintained and re-established in the wild populations.
Contact
Email: John.Gilbey@gov.scot
There is a problem
Thanks for your feedback