Conservation of Atlantic salmon - gene banking: principles and considerations
This report examines the procedures, considerations, risks and opportunities of gene banking for Atlantic salmon conservation and restoration in Scotland. Presented is a brief overview of live gene banking and a detailed focus on cryobanking to preserve gametic material through freezing and storage.
Cryopreservation protocols
There are a number of stages in the cryopreservation of salmon sperm and it is vital that, at each stage, the correct procedures and protocols are followed. These are outlined in Figure 1 and briefly described, in general terms, in the text (after Di Iorio et al. 2023). However, successful cryopreservation requires that, at all stages of this procedure, detailed advice, training and testing are carried out, as well as the development of bespoke protocols in order to ensure successful outcomes (Martínez-Páramo et al. 2017, Bøe et al. 2021, Di Iorio et al. 2023).
Gamete collection
Sperm is collected from a representative selection of fish in the population/s under conservation, as detailed above. Sperm samples are collected by stripping fish into labelled tubes stored on ice. Samples are transferred to the storage facility within a time period of less than ~6 hours (Rusco et al. 2021).
Sperm quality
In order to retain only high-quality samples and thus ensure both the best possible chance of successful preservation, both sperm motility and sperm concentration are evaluated for each sample. Validated protocols are followed for each procedure and only those samples meeting the quality threshold/s are, in normal cases, retained. However, in extremis, where there are very few samples available, it may be the case that all samples are retained with the hope that they will prove viable.
Cryopreservation
Once quality has been assessed, the samples are diluted with a specific freezing medium to reach a final defined concentration when in the storage medium (extender). They are then inoculated into labelled cryopreservation ‘straws’ or other sample tubes which may store a larger quantity of sperm and allow fertilisation of more eggs (e.g. SquarePack® as used in the Norwegian Gene Bank; (Bøe et al. 2021)). Straws are then allowed to equilibrate before being frozen (again, using suitable protocols) with liquid nitrogen and stored in the cryobank (Figure 2). The liquid nitrogen tanks (dewars) are stored in an air-conditioned room with a controlled temperature (~5°C). In order to ensure an appropriate level of liquid nitrogen, the storage dewars should be refilled with liquid nitrogen, at regular intervals. Sperm cryopreserved in this way can be stored almost indefinitely without substantial effect on cell viability or quality (Stoss and Refstie 1983, Bøe et al. 2021).
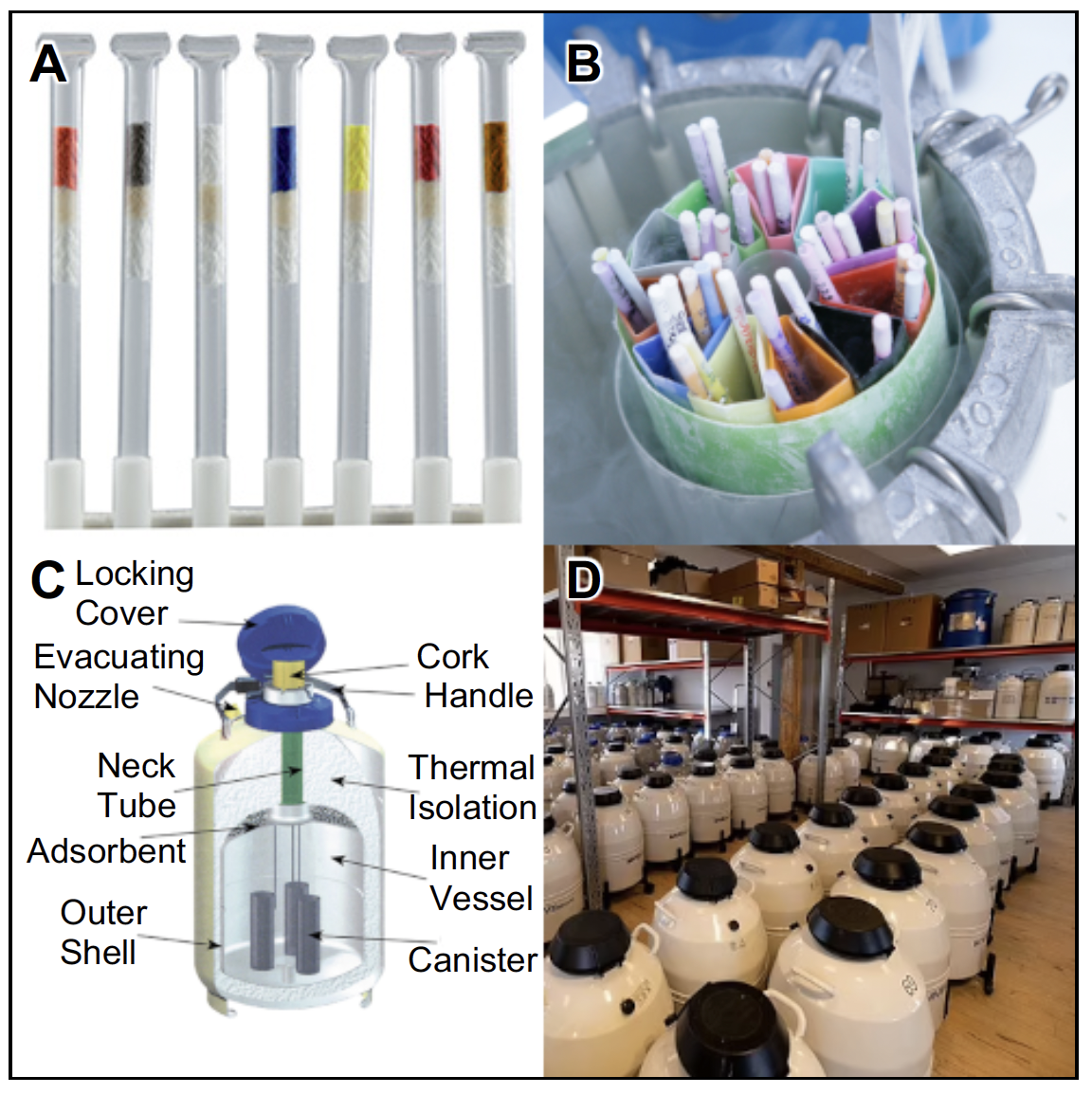
Thawing and use
When required for fertilisation, frozen sperm can be thawed, using appropriate protocols, for use. In order to ensure successful fertilisation, the motility of the sperm is again tested and only samples that are above the required threshold are utilised.
Database management
Together with collecting and storage of samples comes the essential and crucial requirement to ensure thorough record keeping is undertaken. In most cases, this will require the setting up of a database for the cryobank management. Each straw would be uniquely identifiable and the donor information associated with the sample should also be recorded. Such information would include (after Di Iorio et al. 2023):
- donor identification code
- sampling site information (coordinates, river, population ID etc)
- donor phenotype and life history information
- donor genotype
- date of semen collection and freezing
- number of straws for each semen donor
- straw colour/s
- list of alpha-numeric straw code/s
- fresh semen quality of each donor (including volume, concentration, motility and viability)
- frozen/thawed semen quality (motility, viability)
- number of straws used for in vitro analysis and in vivo fertilization
- cryogenic tank ID/s for straw/s
- position in the cryogenic tank/s
- any other useful information (e.g. screening results for farm fish/hybrids)
It is vital that proper database management (and backup) is enacted throughout the lifetime of the cryobank using staff trained to an appropriate level.
Contact
Email: John.Gilbey@gov.scot
There is a problem
Thanks for your feedback