Draft Fisheries Assessment – West of Scotland NCMPA: Fisheries management measures within Scottish Offshore Marine Protected Areas (MPAs)
These assessments look at the fishing activity occurring within each offshore MPA and SAC and assess the potential impacts of this activity on the protected features within each site. This assessment is for West of Scotland NCMPA.
3. Part B Assessment – Fisheries Assessment
3.1 Fisheries assessment overview
Part B of this assessment considers if there would be a risk of the fishing activities identified in Part A, at the levels identified in the relevant date range, hindering the achievement of the conservation objectives for the MPA, in order to consider whether, and if so, which, management measures might be appropriate for the MPA, taking into account all relevant statutory obligations incumbent upon the Scottish Ministers.
The fishing activities and pressures identified in Part A which have been included for assessment in Part B, are demersal trawls, demersal seines, pelagic fishing and anchored nets/lines. The pressures associated with these fishing activities that have been included in Part B are;
- abrasion/disturbance of the substrate on the surface of the seabed;
- penetration and/or disturbance of the substrate below the surface of the seabed, including abrasion;
- smothering and siltation rate changes (Light);
- changes in suspended solids (water clarity);
- removal of non-target species and
- removal of target species.
3.2 Fishing Activity Descriptions
3.2.1Existing management within West of Scotland NCMPA
In compliance with Part 5, Chapter 7 of The Common Fisheries Policy and Aquaculture (Amendment etc.) (EU Exit) Statutory Instrument (S.I.) 2019 No. 753, there is a ban on the use of all bottom-contacting mobile gear below 800 m depth across all UK waters. This applies across the area of West of Scotland NCMPA where the depth falls below 800 m. Part 5 Chapter 7 of S.I. 2019, No. 753 also implements restrictions on fishing between 400 m and 800 m where Vulnerable Marine Ecosystems (VMEs) are present, or are likely to occur. These rules aim to minimise the impact of fishing activities on VMEs. Under The Common Fisheries Policy and Animals (Amendment etc.) (EU Exit) Regulations 2019 S.I. 2019, No. 1312 (amending S.I. 2019, No. 753) there is a prohibition on the use of bottom-set gillnets, entangling nets, and trammel nets at depths greater than 200 m for the protection of deepwater shark species. These protective measures are also applied in the North-East Atlantic Fisheries Commission (NEAFC) technical measures regulatory area (beyond European Union waters) through the same Statutory Instrument. In the areas close to Anton Dohrn Seamount, Rosemary Bank and George Bligh Bank these gillnet restrictions are only seasonal. There are also seasonal restrictions placed in certain areas for blue ling and herring.
3.2.2 Fishing activity within the NCMPA
The West of Scotland NCMPA is a large area that overlaps ICES rectangles 42D6, 42D7, 42D8, 42D9, 42E0, 43D6, 43D7, 43D8, 43D9, 43E0, 44D7, 44D8, 44D9, 44E0, 45D5, 45D6, 45D7, 45D8, 45D9, 45E0, 46D5, 46D6, 46D7, 46D8, 46D9, 46E0, 46E1, 46E2, 47D5, 47D6, 47D7, 47D8, 47D9, 47E0, 47E1, 47E2, 47E3, 48D5, 48D6, 48D7, 48D8, 48D9, 48E0, 48E1, 48E2, 48E3, 49D6, 49D7, 49D8 and 49D9 which cross the Faroe Grounds (ICES Division 5b), West of Scotland (ICES Division 6a) and Rockall (ICES Division 6b), in the Rockall, Hebrides, Bailey, Hatton and North Scotland Coast regions. The main gear types for UK vessels are midwater trawls, demersal trawls and hooks and lines.
The VMS-based estimates and ICES rectangle landings statistics indicate that over-12 m midwater trawls and demersal trawls are the predominant UK vessels that operated within the site over the period 2015-2019.
For the over-12 m vessels, based on the VMS data from 2015-2019, demersal trawls operate predominantly in the southern part of the site, as well as along the eastern arc boundary, with midwater trawls concentrated mainly towards the south-eastern boundary. Set net activity is found mainly in a relatively small area over in the western edge of the site where shallower water persists. The distribution of under-12 m vessels’ effort within these ICES rectangles indicates that landings recorded in these rectangles are unlikely to be taken from within the site itself.
In addition to UK activity, vessels from Norway (80 vessels), Ireland (57 vessels), Faroes (32 vessels), France (26 vessels), Spain (15 vessels), Germany (13 vessels), Denmark (10 vessels), Netherlands (8 vessels), Lithuania (6 vessels) and Poland (number of vessels cannot be disclosed) may also operate in the site, based on the VMS data from 2015-2019. However, it is not clear what gear types these vessels operate, nor whether they were actively fishing at the time.
3.2.3 Demersal trawls
The aggregated gear method of demersal trawls includes multiple gears that operated within the West of Scotland NCMPA between 2015 and 2019. These include bottom otter trawls, multi-rig trawls and other not specified bottom trawl types (Table 1). The target species for these gear types are demersal fish, molluscs or nephrops. Similar pressures are exerted by the different gears used for demersal trawling, subsequently the aggregated gear type of ‘demersal trawl’ was used to map activity across the site.
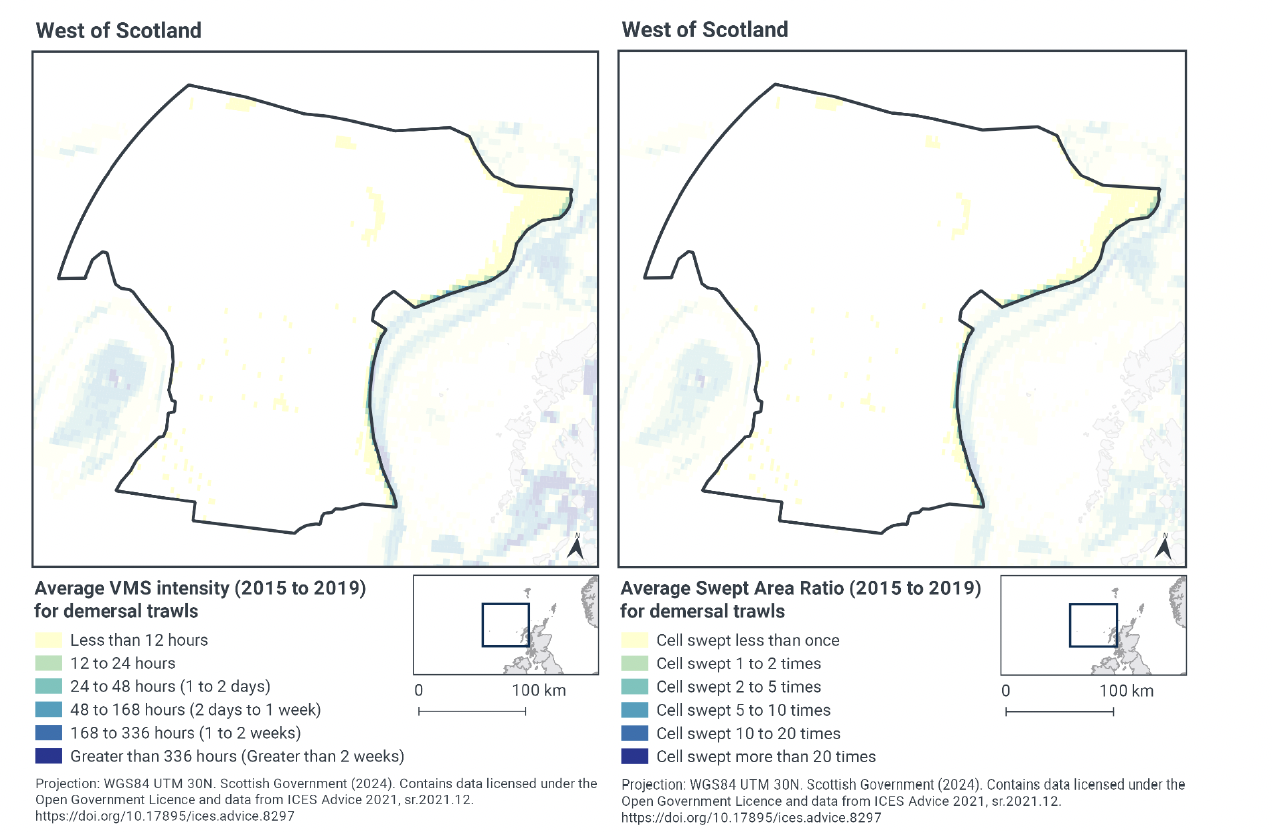
Based on the VMS, demersal trawl activity within West of Scotland NCMPA occurs at relatively low levels. Effort is concentrated along the continental slope, particularly in the north-east of the site, on the topographic features of the seamounts and George Bligh Bank, and also in a small area in the south of the site near Rockall Bank (Figures 1 and 2). Activity within the site boundary peaked at less than 12 hours per year per grid cell between 2015-2019 (Figure 2). Fishing activity tends to be concentrated along the easter boundary of the site (continental shelf edge) and average activity is between 12 to 24 hours per year per grid cell. The remainder of the site has no demersal trawling activity.
Swept-Area Ratio (SAR) information averaged over the same time period shows similar patterns of fishing intensity as the VMS data. Around the topographic features and the eastern boundary of the site (continental shelf edge), cells were swept only once per year between 2015-2019 (Figure 2). Again, evidence of fishing along the boundary of the site is shown where there are small areas of slightly higher SAR information, with cells swept 1-2 times per year. The rest of the site had no SAR values indicating no demersal trawling occurs.
3.2.4 Demersal seines
The aggregated gear method of demersal seines operated within the West of Scotland NCMPA between 2015 and 2019, as shown on the ICES gridded data (Figure 3). It was not possible to identify the specific gear types, however, as data from EU and Norwegian vessels was not available at this level of granularity. The target species for demersal seines are demersal fish.
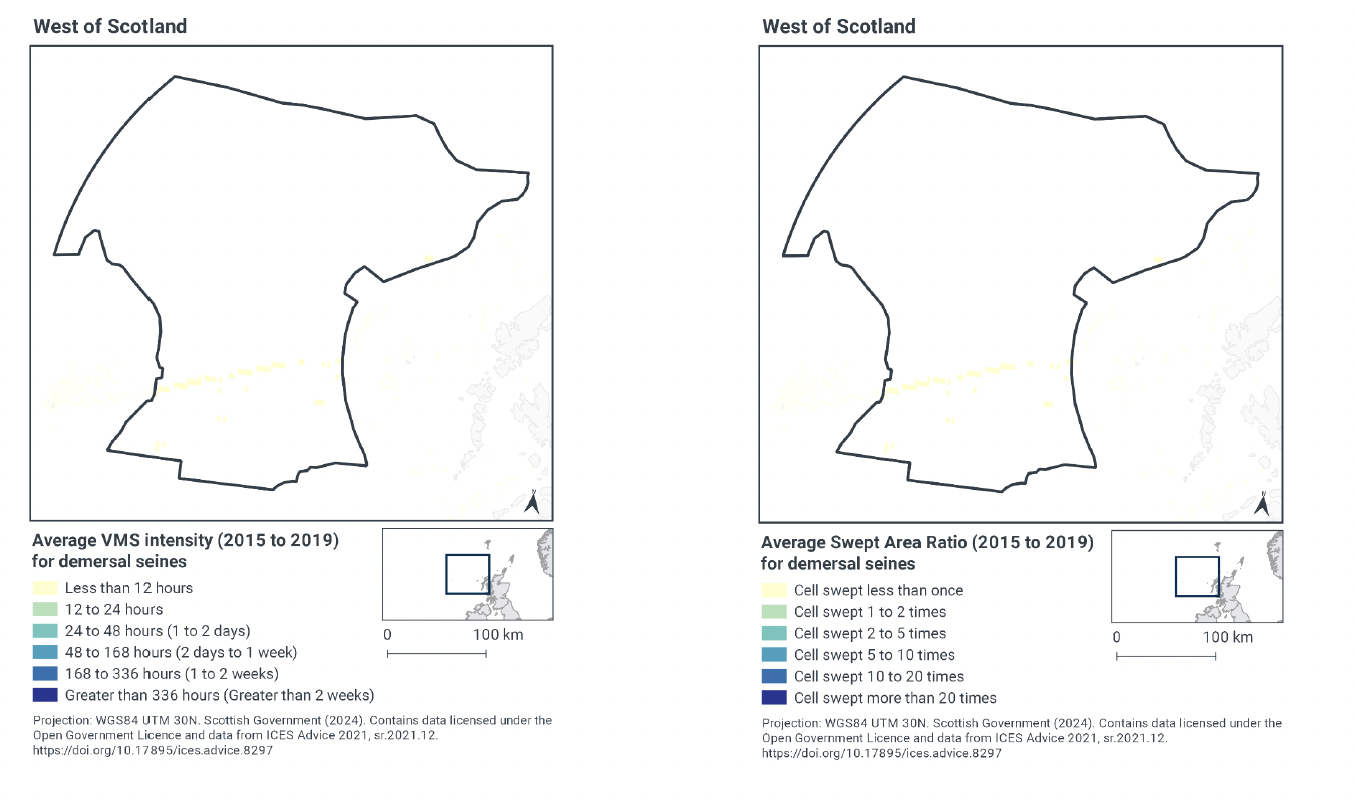
Based on the ICES gridded data, demersal seine activity within West of Scotland NCMPA occurs at relatively low levels. Effort is concentrated in a line across the south of the site, with activity peaking at less than 12 hours per year per grid cell between 2015-2019 (Figure 3). The remainder of the site has no demersal seine fishing activity.
Swept-Area Ratio (SAR) information averaged over the same time period shows similar patterns of fishing intensity as the VMS data. There is a single line of demersal seine activity across the south of the site, where cells were swept less than once per year between 2015-2019 (Figure 3). The rest of the site had no SAR values indicating no demersal seine fishing occurs.
3.2.5 Pelagic fishing
The aggregated gear method of pelagic fishing included mid-water trawls (single) that operated within the West of Scotland NCMPA between 2015 and 2019 (Table 1). The target species for these gear types are pelagic fish.
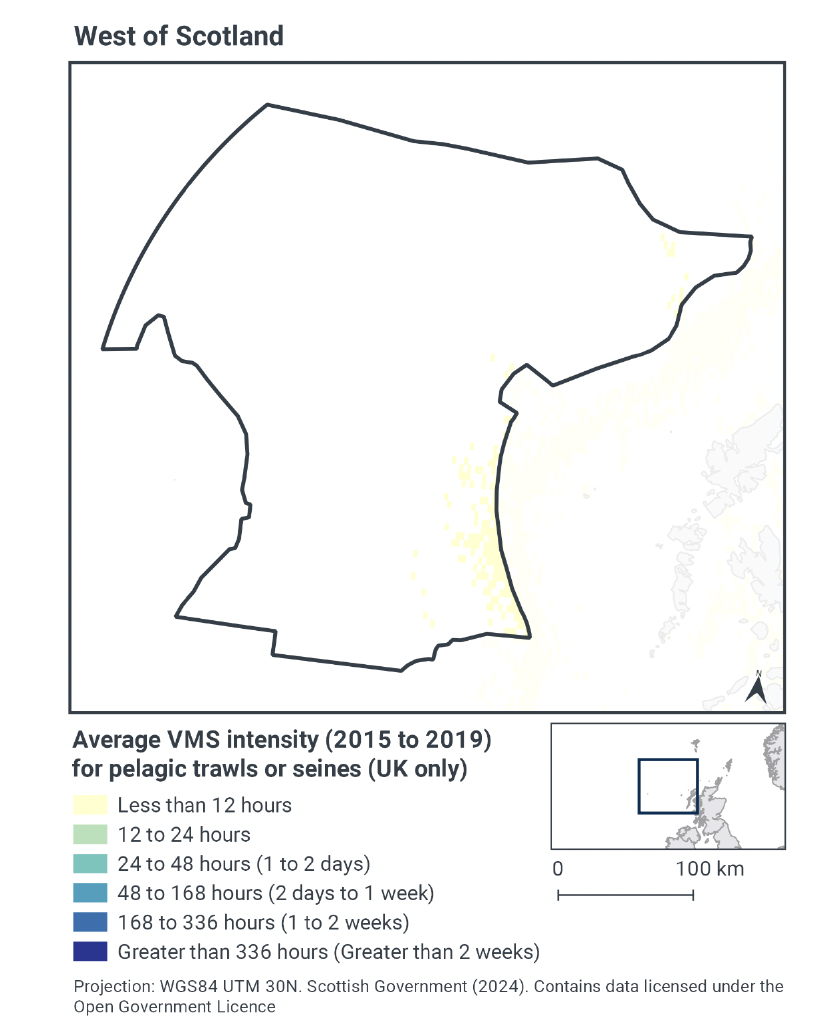
Based on the VMS, pelagic fishing activity within West of Scotland NCMPA occurs at relatively low levels, however there are a number of limitations with the interpretation of the VMS for pelagic fishing estimates that should be considered here. Effort of pelagic fishing is therefore likely to be underestimated from the VMS activity maps (Figure 4) alone. The rationale for this underestimation is due to the operation of the fishing activity being relatively short in comparison to, for example, demersal trawls. There is generally a two hour reporting frequency of the VMS that means the pelagic fishing activity is likely to be under-estimated. In addition pelagic shoals may be fishes in different areas in different years, resulting in average cell values can be low. Lastly this data is for UK vessels only, there is pelagic activity effort by a variety of other nationals along the edge of the continental shelf which the VMS activity cannot be accessed.
Effort is concentrated along the south-eastern edge of the site and in a small area in the north eastern corner, with activity peaking at less than 12 hours per year per grid cell between 2015-2019 (Figure 4). The remainder of the site has no pelagic fishing activity.
3.2.6 Anchored nets/lines
The aggregated gear method of anchored nets/lines includes multiple gears that operated within the West of Scotland NCMPA between 2015 and 2019. These include set gillnets and set longlines (Table 1). The target species for these gear types are demersal fish. Similar pressures are exerted by the different gears used for anchored nets/lines fishing, subsequently the aggregated gear type of ‘anchored nets/lines’ was used to map activity across the site.
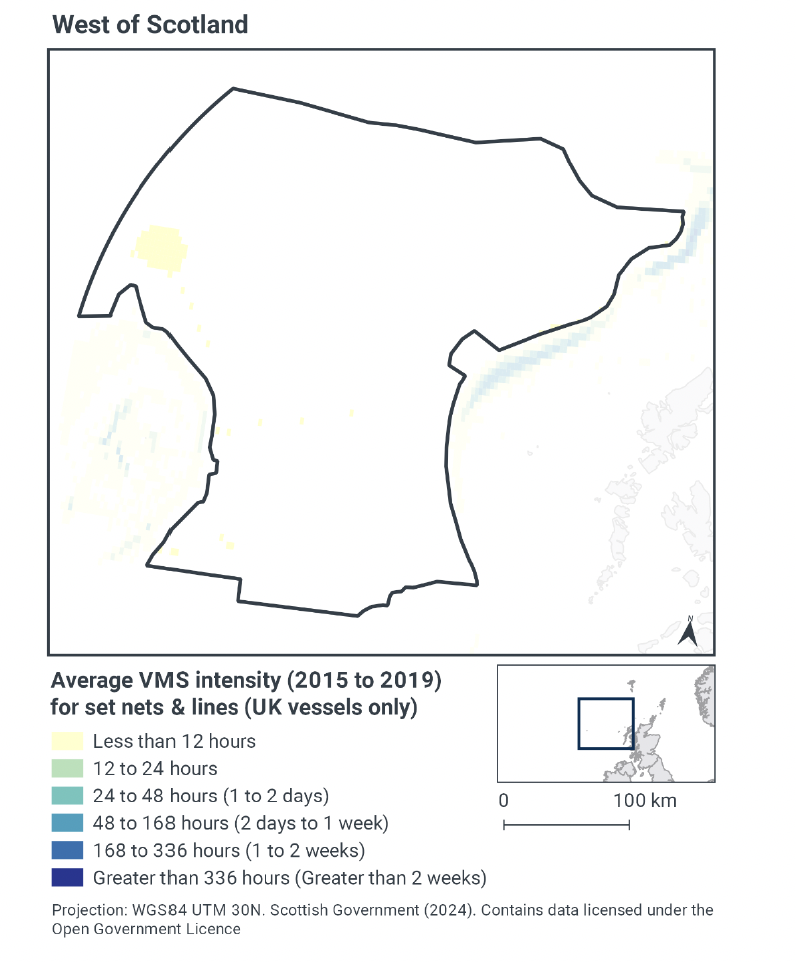
Based on the VMS, anchored nets/lines activity within West of Scotland NCMPA occurs at relatively low levels. Effort is concentrated over the topographic feature of George Bligh Bank, and within a small area in the south of the site near Rockall Bank, with activity peaking at less than 12 hours per year per grid cell between 2015-2019 (Figures 1 and 5). The remainder of the site has no anchored nets/lines fishing activity.
3.2.7 Summary of fishing activity within West of Scotland NCMPA
Fishing activity within the West of Scotland NCMPA is relatively low, with only limited demersal trawl, demersal seine, pelagic and anchored nets/lines activity occurring. Where activity does occur, it appears to be concentrated around the topographic features of the site, such as the seamounts, and/or along the continental shelf edge (eastern boundary of the site). These low levels of activity for demersal mobile and static gear are reflective of the existing management measures that are already in place within the site, as described in Section 3.2.1 above.
3.3 Fishing activity effects overview
The following sections explore the pressures associated with fishing activity (demersal trawl, demersal seine, pelagic fishing and anchored nets/line) within the West of Scotland NCMPA that were considered capable of impacting the protected features. The pressures considered are:
- Abrasion/disturbance of the substrate on the surface of the seabed;
- Penetration and/or disturbance of the substrate below the surface of the seabed, including abrasion;
- Smothering and siltation rate changes (Light);
- Changes in suspended solids (water clarity);
- Removal of non-target species and
- Removal of target species.
These six pressures were exerted by a combination of demersal trawl, demersal seine, pelagic fishing and anchored nets/line, and were considered capable of impacting the protected features.
Given the similarity between ‘abrasion/disturbance of the substrate on the surface of the seabed’ and ‘penetration and/or disturbance of the substrate below the surface of the seabed’, these two pressures are considered together in the text below.
Information on the sensitivity of the protected features to each of these pressures is presented below and is taken from FeAST, the West of Scotland NCMPA Fisheries Management Options Paper and the feature specific fisheries management guidance prepared by JNCC and NatureScot. At the end of this section, a summary of the overall potential impacts associated with the demersal trawls, demersal seines, pelagic fishing and anchored nets/lines fishing activity is presented.
3.3.1 Impacts of demersal mobile gear (trawls and seines) on habitat features
The species associated with Seamount communities tend to be composed of erect and fragile species that are sensitive to physical disturbance, particularly deep-sea stony corals, gorgonians and black corals, sea anemones, hydroids and sponges (Clark et al. 2010; Clark and Tittensor, 2010). Significant reductions in stony coral cover and associated species abundance and diversity have been observed on trawled seamounts in New Zealand and Australia (Goode et al. 2020). Clark and Tittensor (2010) found that roughly 100 trawl tows can reduce coral to very low mean levels (<1%) on New Zealand seamounts. Between approximately 100 and 800 tows would remove coral cover entirely. However, mean coral cover on some seamounts can be reduced to less than 1% with far fewer tows. Single passes of trawls can themselves cause more than half of sponges and corals present to be visibly damaged (Freese et al. 1999). Mortality of species can occur both by disturbance at the seabed from trawls or through being brought to the surface, resulting in a reduction in abundance (ICES, 2010; Jennings and Kaiser, 1998; Kaiser and Spencer, 1996).
Despite some seamount taxa being more resistance to the direct effects of bottom-trawling, Goode et al. (2020) concluded that seamount benthic communities overall appear to have low resistance. Recovery from damage is estimated to be measured in decades, depending on the environmental conditions and biological variables, although the species present on seamounts can exhibit varying recovery rates (ICES, 2010; Clark et al. 2010; Goode et al. 2020). Species with higher longevity, such as habitat-forming corals and sponges, take much longer to recover. As these can form a key part of Seamount communities, any impacts to those species can significantly alter the structure and function of the Seamount communities feature (Goode et al. 2020). These features (Deep-sea sponge aggregations, Cold-water coral reefs and Coral gardens), which are also protected in their own right within the West of Scotland NCMPA, are discussed below.
There is no evidence of impacted Seamount communities regaining their pre-disturbance condition in terms of community composition, megafaunal abundance or species diversity (Goode et al. 2020), indicating the importance of management prior to impacts occurring where possible. Based on the evidence above, there is a high risk that mobile bottom contact gear will affect the extent and distribution of Seamount community features, as well as their structure and function.
Deep-sea sponge aggregations are highly sensitive to bycatch, abrasion, and penetration pressures (Dinwoodie, 2021a, 2021b, Last et al. 2019a, 2019b). Studies on Deep-sea sponge aggregations have found that trawling damages, displaces and removes sponges through direct physical impact, as well as from disturbed sediment resettling and causing smothering beyond the path of the trawl itself (Buhl-Mortensen et al. 2016; ICES, 2007, 2010; Kędra et al. 2017; OSPAR, 2010a). Deep-sea sponges have some capacity for recovery from mild damage, but significant disturbance, damage or smothering may result in sponges being unlikely to survive (Fang et al. 2018; Freese, 2001; ICES, 2007, 2010; Jones et al. 2012; Malecha and Heifetz, 2017). Pham et al. (2019) modelled the impact of bottom trawling on sponge grounds dominated by Geodia sp. in Canadian waters, finding that a simulation of 30 trawls would remove 884 tonnes of sponges. Similarly, a scientific experiment on the effects of an Agassi bottom trawl on deep-sea sponge grounds in the Arctic Ocean significantly reduced megafaunal densities, including large sponge species (Morrison et al. 2020). Although smaller morphotype sponges showed lower trawling impacts, it is the large sponges that have the greatest contribution to the structural complexity of Deep-sea sponge aggregations (Morrison et al. 2020). In addition to reductions in numbers, Geodia spp. sponges in areas impacted by trawling may also have reduced mean individual sponge biomasses (Kędra et al. 2017). Viera et al. (2020) inferred a relationship between increased bottom trawl fishing activity and decreased aggregation-forming sponge Pheronema carpenteri condition (individual mass, sponge equatorial diameter, and geometric mean densities). Morrison et al. (2020) found no signs of recovery of impacted deep-sea sponge grounds four years after the trawling occurred, whilst Malecha and Heifetz (2017) found significant damage to sponges evident in the deep-sea sponge communities after 13-years following trawling impact. Sedimentation events, which can also be caused by trawling activity, similarly resulted in negligible recovery over a 10-year period (Jones et al. 2012). Recovery of structure and function following damage is therefore likely to take at least 25 years (Dinwoodie, 2021a, 2021b, Last et al. 2019a, 2019b). Deep-sea sponge aggregations dominated by Geodia spp. play a key functional role in the wider deep-sea environment, filtering approximately 56,000 million litres of seawater on a daily basis, consuming roughly 63 tonnes of organic carbon through respiration and contributing to the turnover of several nitrogen nutrients (Pham et al. 2019). Based on the evidence above, there is a high risk that mobile bottom contact gear will affect the extent and distribution of Deep-sea sponge aggregation features, as well as their structure and function.
Cold-water coral reefs are highly sensitive to bycatch, abrasion and penetration pressures (Garrard et al. 2020). Bottom trawling has been found to severely damage reefs, breaking up the structure, fragmenting the reef, and potentially resulting in the complete disintegration of the coral matrix, and loss of the associated species (Fosså et al. 2002; Grehan et al. 2005; Hall-Spencer et al. 2002; Roberts et al. 2009; Rogers, 1999). Cold-water coral specimens can also be bycaught in trawls (Durán Muñoz et al. 2012). Cold-water coral reefs can occur on seamounts, and as stated above, significant reductions in stony coral cover and associated species abundance and diversity have been observed on trawled seamounts in New Zealand and Australia (Goode et al. 2020). Clark and Tittensor (2010) found that roughly 100 trawl tows can reduce coral to very low mean levels (<1%) on New Zealand seamounts. Between approximately 100 and 800 tows would remove coral cover entirely. However, mean coral cover on some seamounts can be reduced to less than 1% with far fewer tows. Cold-water coral reef habitats completely damaged by physical pressures such as those associated with benthic trawling do not show signs of recovery even a decade after such pressure has been removed (Althaus et al. 2009; Buhl-Mortensen et al. 2013; Buhl-Mortensen, 2017; Hall-Spencer et al. 2002; Howell et al. 2014; Huvenne et al. 2016; Williams et al. 2010). However, recovery (or regrowth) has been observed in areas where some living coral remains after impact (Buhl-Mortensen et al. 2013; Buhl-Mortensen, 2017; Huvenne et al. 2016). If coral colonies are killed, any recovery of extent and distribution will be influenced by the method of reproduction, dispersal potential, the relative location of a potential source population of reproductive adults and the presence of suitable supporting habitat (Dahl et al. 2012; Fox et al. 2016). Evidence indicates that for some species of cold-water corals, successful recruitment events may only occur once a decade (Stone et al. 2015), which could limit the opportunities for recovery. Based on the evidence above, there is a high risk that mobile bottom contact gear will affect the extent and distribution of Cold-water coral reef features, as well as their structure and function.
Coral gardens are highly sensitive to physical disturbance and bycatch (Yoklavich et al. 2018). Mobile benthic gears can result in significant damage and mortality (Durán Muñoz et al. 2012; OSPAR, 2010b) and over time, the structural and biological diversity of the habitat will be reduced. Coral gardens on soft bottoms within fishing depths are particularly vulnerable (Edinger and Sherwood, 2009), however, where they occur on low relief hard substrate Coral gardens may also be accessible to rockhopper gears (OSPAR, 2010b). Re-establishment of individual specimens of corals may occur within 50 to 100 years but the time taken for complex coral garden habitat to develop is likely to be longer (ICES, 2010). Based on this evidence, there is a high risk that mobile bottom contact gear will affect the extent and distribution of Coral garden features, as well as their structure and function.
In lower energy deep water locations, such as in the West of Scotland NCMPA, sedimentary habitats tend to be more stable and their associated fauna less tolerant of disturbance (Hiddink et al. 2006; Kaiser et al. 2006). Studies have shown that areas of mud habitats (which includes Offshore deep-sea mud and Burrowed mud including sea-pens) subject to mobile fishing activity, support a modified biological community with lower diversity, reduction or loss of long-lived filter-feeding species and increased abundance of opportunistic scavengers (Ball et al. 2000; Tuck et al. 1998). This effect is often greatest in the more heavily fished offshore areas suggesting that impact is related to the intensity of fishing (Ball et al. 2000). Furthermore, modelling studies suggest that the greatest impact is produced by the first pass of a trawl (Hiddink et al. 2006). Trawling on these deep-sea sedimentary habitats can cause significant decreases in organic matter content, slower organic carbon turnover, reduced meiofauna abundance, biodiversity and nematode species richness (Pusceddu et al. 2014). The use of penetrative gear over soft substrates, can further cause removal or re-stratification of sediment layers and homogenisation of sedimentary habitats (Goode et al. 2020; Martín et al. 2014). Sediment resuspension can also occur, resulting in increases in turbidity and risks of smothering to benthic fauna (Martín et al. 2014). The physical integrity of the seabed can also be altered, becoming flattened in trawled areas with less bioturbation (fewer and smaller burrows, mounds and faunal tracks) compared to non-trawled areas (Ramalho et al. 2017). Other physical impacts include scars created by the trawl doors (Goode et al. 2020). These alterations to the seafloor structure can be long lasting, with scars remaining visible for more than 10 years after trawling ceases (Goode et al. 2020). Based on the evidence above, it is likely that mobile bottom contact gear will affect the extent and distribution, and structure and function of Burrowed mud (including sea-pens) and Offshore deep-sea mud features, including the sediment composition and finer scale topology. Such sedimentary habitats also provide an important blue carbon store and although the interaction between mobile fishing gear and sediments is complex (Epstein et al. 2021), research has shown that the west coast of Scotland is a key area where sedimentary carbon is potentially at greatest risk from bottom trawling activity (Black et al. 2022).
Deep-sea sea-pens, associated with Burrowed mud and Offshore deep-sea mud habitats, are likely to have medium sensitivity to bycatch, abrasion and penetration pressures and are highly sensitivity to heavy levels of smothering (up to 30cm) (Last et al. 2020a, 2020b). Although some sea-pen species have behavioural adaptations and can recover from minor damage (Kenchington et al. 2011; Malecha and Stone, 2009; Troffe et al. 2005), high levels of bycatch in trawl nets can occur and incidental mortality is a concern for those remaining on the seafloor (Last et al. 2020a, 2020b). Otter trawls have been found to catch the greatest frequency of sea-pens compared to other gear types, e.g., twin trawl, triple trawl, shrimp trawl, and static gears (Wareham and Edinger, 2007). Dredges can also catch high numbers of sea-pens (Pires et al. 2009). A number of studies indicate that the abundance of sea-pen species are negatively correlated with bottom trawling (Adey, 2007; Buhl-Mortensen et al. 2016; Hixon and Tissot, 2007). In addition to sea-pens, Nephrops may be an important component of the benthic community associated with Offshore deep-sea mud and Burrowed mud. Any fisheries, such as mobile bottom-contact gears, that greatly alter the abundance or size composition of this species may therefore have a negative impact on the biological structure of the features. This evidence further suggests that mobile bottom contact gear will likely affect the biological assemblages and biological structure of the features, resulting in impacts to the extent and distribution, and the structure and function of the Burrowed mud (including sea-pens) and Offshore deep-sea mud habitat features.
Similar to the above, trawling on Offshore sands and gravels also can cause significant decreases in organic matter content, slower organic carbon turnover, reduced meiofauna abundance, biodiversity and nematode species richness (Pusceddu et al. 2014). Stable Offshore sands and gravels often support a ‘turf’ of fragile species which are easily damaged by trawling and recover slowly (Collie et al. 2005; Foden et al. 2010). Trawling and dredging tends to cause increased mortality of fragile and long lived species and favour opportunistic, disturbance-tolerant species (Bergmann and Van Santbrink, 2000; Eleftheriou and Robertson, 1992). Some particularly sensitive species may disappear entirely (Bergmann and Van Santbrink, 2000). The net result is benthic communities modified to varying degrees relative to the un-impacted state (Bergmann and Van Santbrink, 2000; Kaiser et al. 2006). The use of penetrative gear over soft substrates, can further cause removal or re-stratification of sediment layers and homogenisation of sedimentary habitats (Goode et al. 2020; Martín et al. 2014). Sediment resuspension can also occur, resulting in increases in turbidity and risks of smothering to benthic fauna (Martín et al. 2014). Other physical impacts include scars created by the trawl doors and dislodgment or removal of boulders, rocks and biogenic substrates (Goode et al. 2020). These alterations to the seafloor structure can be long lasting, with scars remaining visible for more than 10 years after trawling ceases (Goode et al. 2020). Based on this evidence, it is likely that mobile bottom contact gear will affect the extent and distribution, and structure and function of Offshore sands and gravels, including the sediment composition, finer scale topology, biological assemblages, and biological structure.
Activity from demersal trawling and demersal seines within West of Scotland NCMPA occurs at relatively low levels and over a very limited spatial scale, and existing management is in place which already restricts the activity permitted within the site (see Section 3.2.1 above). However, the protected habitat features of the site, in particular Deep-sea sponge aggregations, Coral gardens, Cold-water coral reefs (including Lophelia pertusa reefs) or Seamount communities, as described above, are highly sensitive to demersal mobile gear activity.
Given the evidence above, the impacts of mobile demersal gear (including demersal trawls and demersal seines) alone within West of Scotland NCMPA at current levels of activity carry a risk of hindering the restoration of the protected habitat features offshore sands and gravels, burrowed mud, offshore deep-sea muds, deep-sea sponge aggregations, coral gardens, cold-water coral reefs (including Lophelia pertusa reefs) or seamount communities, such that the extent and distribution, structure and function and supporting processes are maintained or restored. Accordingly, Scottish Ministers conclude that demersal mobile gear alone are capable of impacting the protected features and, at current levels, would or might hinder the achievement of the conservation objectives of the MPA.
3.3.2 Impacts of anchored nets/lines fishing on habitat features
No studies providing evidence of the effects of static gears on Scottish seamount communities were found, however impacts occurring on analogous vulnerable habitats and species, such as sponges and corals in Scottish waters are applicable (Durán Muñoz et al. 2011). Impacts can arise from hooks, lines, nets and ropes becoming entangled with corals and other fragile species, including ‘plucking’ them from the seabed during hauling (Durán Muñoz et al. 2011; Mortensen et al. 2005; OSPAR, 2010b). While the degree of damage from individual fishing operations is likely to be lower than for trawling, cumulative damage may be significant. Based on the evidence above, there is a high risk that static bottom contact gear will affect the extent and distribution of Seamount community features, as well as their structure and function.
The Deep-sea sponge aggregation feature is considered to be sensitive to static gear activity, notably because sponges may become caught or entangled in static gears and damaged on the seabed or brought to the surface (OSPAR, 2010a). Such by-catch by demersal longliners of hexactinellid and demospongid sponges has been documented within the North-east Atlantic (Durán Muñoz et al. 2011), the Azores (Cyr, 2018) and in the Antarctic (Parker and Bowden, 2010). One study on Hatton Bank collected 3.5 kg of sponges from a total of 38 longline sets (Durán Muñoz et al. 2011), however this only contributed < 0.1% of the total catch: 65.8% of the total sponge catch was obtained with monofilament gear, compared to 34.2% with multifilament gear. In the Azores, low bycatch rates were recorded overall (0.07 sponge per 1000 hooks), however on average per 1000m2, 1 out of 4 individuals remaining on the seafloor were left damaged by the longline activities (e.g., fragmented, dislodged, entangled or dead; Cyr, 2018). These in-situ impacts, causing incidental mortality and abrasive damages, were greater for sponges with higher structural complexities, such as those with massive, flabellate and pedunculate morphologies (Cyr, 2018). Where sponges are dislodged, this is likely to impact a sponge’s ability to filter water (Parker and Bowden, 2010). While these evidence source show that the extent of damage caused by individual static gear fishing events is likely to be lower than that for trawling, the effect of cumulative damage may be significant. Recovery from damage is likely to take at least 25 years (Dinwoodie, 2021a, 2021b, Last et al. 2019a, 2019b). Based on the evidence above, particularly considering cumulative effects, there is a high risk that static bottom contact gear will affect the extent and distribution of Deep-sea sponge aggregation features, as well as their structure and function.
Damage to Cold-water coral reefs and Coral gardens can occur from static fishing gear such as gill nets and long-line fisheries, where corals can become entangled in ropes/lines or nets and can be plucked off the seabed during hauling (Fosså et al. 2002; ICES, 2010; Mortensen et al. 2005; OSPAR, 2010b; Parker and Bowden, 2010; Wareham and Edinger, 2007). Bottom longlining poses a high risk to large erect species such as gorgonians, cup corals, soft corals, black corals and lace corals (Durán Muñoz et al. 2011; OSPAR, 2010b). In a study off Portugal, 85% of bottom-set gillnet deployments caught cold-water corals, 45% of which were entire colonies and overall 22 different coral species were recorded as bycatch (Dias et al. 2020). Coral bycatch was higher when the nets were deployed on or nearby areas where rocky substrate is known to occur. The average coral CPUE was 0.92 per day with a 100 m net length (31.1 corals per set), however this increased to 13.02 over rocky substrates. A study in the Ionian Sea similarly found that 72% of longline sets captured corals (Mytilineou et al. 2014). In comparison, in the Azores, Sampaio et al. (2012) reported that 15.2% of 297 commercial longline fishing trips landed corals and deep-sea longline fishing removed 0.32 corals per 1000 hooks (1.14 corals per set; Pham et al. 2014). Where static gears do cause mortality or damage to coral garden habitats, the recovery and re-establishment characteristics are the same as those for mobile gears above. Traps are unlikely to catch any bycatch in comparison (Shester and Micheli, 2011). It is worth noting that these coral removal rates are much lower than those reported for bottom trawling (Clark et al. 2016). Site specific difference in coral density will also affect the bycatch rates. Based on the evidence above, there is a high risk that static bottom contact gear will affect the extent and distribution of Cold-water coral reef and Coral garden features, as well as their structure and function.
Offshore sands and gravels within subtidal areas are not considered to be sensitive to the level of abrasion caused by static demersal gears, with minimal impact on the faunal communities and seabed structure (Tillin et al. 2010; Tyler‐Walters et al. 2009). However, in lower energy deep water locations, such as in the West of Scotland NCMPA, sediments tend to be more stable and their associated fauna less tolerant of disturbance (Hiddink et al. 2006; Kaiser et al. 2006). Bycatch of associated communities, such as invertebrates also poses a risk. Overall, the risk from low levels of static bottom contact gear on the abundance and distribution, and the structure and function of Offshore sands and gravels is likely to be limited, however higher levels of fishing activity will pose a greater risk to the features and their attributes.
Bycatch of deep-sea sea-pen species (associated with Offshore deep-sea mud and Burrowed mud) has been recorded in gillnets and longlines, although at a lower frequency than otter trawls (Wareham and Edinger, 2007). Longline hooks of varied sizes can catch specimens of all size ranges, including larger specimens (de Moura Neves et al. 2018). If static fishing activity is low, direct impact on the habitat is likely to be minimal and seabed structure is likely to be maintained in a slightly modified state (Adey, 2007). In addition to sea-pens, Nephrops may be an important component of the benthic community associated with Offshore deep-sea mud and Burrowed mud. Any fisheries, such as static gears, that greatly alter the abundance or size composition of this species may therefore have a negative impact on the biological structure of the features. Based on the evidence above, the risk from low levels of static bottom contact gear on the abundance and distribution, and the structure and function of Burrowed mud (including sea-pens) and Offshore deep-sea mud is likely to be limited, however higher levels of fishing activity will pose a greater risk to the features and their attributes.
Activity from anchored nets/lines within West of Scotland NCMPA occurs at relatively low levels and over a very limited spatial scale, and existing management is in place which already restricts the activity permitted within the site (see Section 3.2.1 above). However, the protected habitat features of the site, in particular deep-sea sponge aggregations, coral gardens, cold-water coral reefs (including Lophelia pertusa reefs) and seamount communities, as described above, are highly sensitive to anchored nets/lines activity.
Given the evidence above, the impacts of anchored nets/lines alone within West of Scotland NCMPA at current levels of activity carry a risk of hindering the conservation objectives of the protected habitat features deep-sea sponge aggregations, coral gardens, cold-water coral reefs (including Lophelia pertusa reefs) or seamount communities. Anchored nets/lines are capable of impacting the burrowed mud, offshore deep-sea mud features but would not hinder the achievement of the conservation objectives of the site at current levels of activity.
Accordingly, Scottish Ministers conclude that anchored nets/lines alone are capable of impacting the protected features; deep-sea sponge aggregations, coral gardens, cold-water coral reefs (including Lophelia pertusa reefs) or seamount communities, and at current levels, would or might hinder but would or might hinder the achievement of the conservation objectives of the NCMPA.
3.3.3 Impacts of demersal mobile gear on mobile species features
Orange roughy (Hoplostethus atlanticus) occurs in a depth band between 180-1800 m (Priede, 2019), corresponding with an area about 20 nautical miles (nm) wide in the West of Scotland NCMPA. The species has historically been targeted in a directed demersal otter trawl fishery in deep water west of Scotland, which resulted in a strong decline in the stock (ICES, 2019a, 2020a). This fishery targeted the spawning aggregations that occur around steep slope and seamount environments, allowing very large catches to be taken over a short period of time, leading to local depletions (FeAST, 2013). However, since 2003 no direct fishery has been permitted for Orange roughy, with limited bycatch allowed in mixed fisheries until 2010 when a zero Total Allowable Catch (TAC) was implemented across all ICES subareas. In addition to the spawning aggregations around seamounts and steep slopes, Scottish deep-water trawl surveys found several juvenile cohorts were present on the gentle slopes of the continental slope (Dransfeld et al. 2013; ICES, 2019a). The species’ long life-span, slow growth rate, late maturity (27.5 years; Minto and Nolan, 2006), low fecundity and episodic recruitment characteristics contribute to its vulnerability, making the species particularly susceptible to population declines if mature adults are removed (Dransfeld et al. 2013). Fishing pressure can also disrupt the schooling behaviour of Orange roughy (Clark and Tracey 1991, cited in Branch, 2001). In areas where fishing is prohibited, smaller and denser aggregations have been observed (Clark et al. 2000). Based on the evidence above, mobile bottom contact gear may affect the presence and distribution of the Orange roughy feature, due to the risk associated with accidental bycatch.
The Roundnose grenadier (Coryphaenoides rupestris) is typically a bottom-living, demersal fish, occurring at depths from 180-2,600 m (Priede, 2019). The species is known to move seasonally up and down the continental slope (Cohen et al. 1990). They are also poor swimmers, so are vulnerable to target and non-target fisheries (Simpson et al. 2011). The long tapering tail of the Roundnose grenadier is also easily damaged after trawling (Priede, 2019; Simpson et al. 2011), suggesting that bycatch incidents can be fatal. The species was first targeted in the North Atlantic by deep-sea fishing fleets in the 1960s and landings peaked in the early 1970s, before declining sharply (Devine and Haedrich, 2008; Priede, 2019). Over a 26-year period from 1978-2003, there was a 99.6% decline in the relative abundance of Roundnose grenadier in the Canadian waters of the northwest Atlantic, as sampled through scientific surveys (Devine et al. 2006). Over 17-years (1978-1994), the individual mean size of Roundnose grenadier declined by 54.9% (Devine et al. 2006). These declines were found to be best explained by fisheries selection, although large-scale atmospheric conditions also played a role (Devine et al. 2006). Catches of Roundnose grenadier in the Rockall Trough have previously represented 28% of entire fish hauls (Mauchline and Gordon, 1984) and on the Hatton Bank the species represented 64% of the catch composition, indicating that the species is at high risk of exploitation. High discards have also been recorded due to catches being comprised of small sized individuals, representing up to 50% of the catch by number and 30% by weight (Durán Muñoz et al. 2012; Pawlowski and Lorance, 2014). Bycatch of Roundnose grenadier most notably occurs in demersal trawl fisheries targeting Greenland halibut, Reinhardtius hippoglossoides and redfish, Sebastes spp. (Devine and Haedrich, 2008; Devine et al. 2006; Jørgensen et al. 2014). Assuming a fisheries catch equal to 5% of the total population, recovery rates of the Roundnose grenadier (based on life history characteristics) are estimated to be between 16 and 136 years (Baker et al. 2009). All size classes are found within the West of Scotland NCMPA (Priede, 2019), so there is a risk of a decline in the mean size of individuals, in addition to there being high discard rates of the smaller individuals. Although there is currently a zero TAC in place for Roundnose grenadier within ICES area 6, based on the evidence above, mobile bottom contact gear may affect the presence and distribution of the Roundnose grenadier feature, due to the risk associated with accidental bycatch.
Blue ling (Molva dypterygia) occur at 500 to 1,250m depths in the Rockall Trough (Priede, 2019) and all Blue ling in the ICES subareas 5b, 6 and 7 (including the whole West of Scotland area) are deemed to be mature (Lorance, 2020). The species has mainly been targeted during their spawning season, due to higher catchability, using standard deep-water trawling techniques, gillnets and longlines (FeAST, 2013). From 1970 to 1990, the bulk of the fishery for Blue ling was seasonal fisheries targeting these aggregations (Lorance, 2020). This has previously led to local depletions of aggregations and in 2009 a seasonal closure (1st March to 31st May each year) was introduced to protect spawning aggregations. Outside the spawning season Blue ling is taken in mixed trawl fisheries (targeting shelf species such as saithe, hake, monkfish and megrim; Lorance, 2020). ICES (2018) found that the spawning-stock biomass has increased since 2004 and the fishing mortality has decreased since 2004. Blue ling recruitment is thought to be stable. In 2017, 95% of landings in ICES subareas 6-7 were in trawl fisheries, with 5% longline fisheries. Discards are thought to be negligible as no undersized Blue ling are caught, and due to low fishing activity, catches have been lower than TACs. Based on the evidence available, a precautionary approach is recommended as there is a risk that the presence and distribution of Blue ling would be impacted if mobile bottom contact gear activity increases.
Evidence for the three deep-sea shark species features, Gulper shark (Centrophorus granulosus), Leafscale gulper shark (Centrophorus squamosus) and Portuguese dogfish (Centroscymnus coelolepis) are presented together below. Literature reviews by Wilson et al. (2009) and Kyne and Simpfendorfer (2007) suggest many long-lived deep-water shark species are unable to self-sustain populations at catch rates exceeding 5% of total biomass. The populations are therefore likely to continue to decline for as long as the species are targeted or taken as bycatch (OSPAR, 2010c). Due to their life history characteristics of very slow growth rates, late maturity, low reproductive potential, long intervals between litters and extreme longevity (Priede, 2019), deep-sea shark species are likely to be very slow to recover (exceeding 25 years), even if deep-water fisheries and all bycatch ceases. There are not known to be any measures that could mitigate the bycatch of sharks in commercial deep-water fisheries, therefore preventing mortality will be very difficult or impossible to achieve whilst fisheries continue in deep-water shark habitats (OSPAR, 2010c). OSPAR (2010d) recommended that a zero by-catch TAC is introduced, but also that bycatch is minimised through depth and effort restrictions, gear controls and area closures, as appropriate. Furthermore, they recommended restricting overall fishing effort in deep-water shark habitat to the lowest possible level.
Gulper shark, Leafscale gulper shark and Portuguese dogfish have historically been landed as bycatch in the mixed deep-water bottom trawl fisheries targeting Roundnose grenadier, Blue ling, black scabbardfish and Orange roughy off the west of Scotland (Priede, 2019), which resulted in significant population declines. In the 1998-2004, a scientific deep-water trawl survey dataset collected by Fishery Research Services (FRS) Marine Laboratory within the 1,200 m depth band (i.e., middle of the species’ depth range), found that population declines were evident for Portuguese dogfish and Leafscale gulper shark (Jones et al. 2005a). Peak catch rates for these species were found to be 62-99% lower compared to pre-fishery values. In 1975, 72% of hauls by Scottish Association for Marine Science surveys in the North-East Atlantic contained at least one Portuguese dogfish specimen, but this declined to 12% in 1999 (OSPAR, 2010d). A bycatch only TAC for deep-sea sharks (including Gulper shark, Leafscale gulper shark and Portuguese dogfish, amongst other species) was introduced in 2007, which was then reduced annually until it became zero in 2010 (ICES WGEF, 2020). No directed fisheries were permitted under these quotas and the landings subsequently declined sharply (Priede, 2019). Between 2009 and 2017, Scottish deep-water survey data has shown no trend in the abundance for Portuguese dogfish (ICES WGEF, 2019). Data from the Scottish deep-water bottom trawl surveys in ICES subarea 6 at depths from 300-2040 m showed a decreasing trend from 2005 to 2011 for Leafscale gulper shark, however abundance has increased and stabilized between 2011 and 2017 (ICES WGEF, 2019).
In general, sharks tend to be fast swimmers so catch rates will be strongly influenced by fishing gear characteristics. Small trawls on a single warp at low speed will be less efficient at catching sharks, compared to larger paired warp trawls used by commercial vessels (Gordon and Swan, 1997; Jones et al. 2005b). However, evidence shows that the deep-sea shark species features are nonetheless at risk from bycatch in the West of Scotland NCMPA. On average, Portuguese dogfish and Leafscale gulper sharks were respectively caught as bycatch in 11% and 15% of deep-water trawl hauls taken by French vessels in the Northeast Atlantic (subareas 4-14) during 2005-2014 (ICES WGEF 2017, Table 3.6). Discards of Portuguese dogfish and Leafscale gulper shark from the fleet in 2018 were estimated to be 172 tonnes, with the majority, if not all of this being from the west of Scotland (ICES WGEF, 2020). In contrast, Portuguese dogfish discards data from Irish trawl fleets operating in the area since 2009 was recorded as being negligible (<1 tonne most years; ICES WGEF, 2020). The 2020 report by the ICES Working Group on Bycatch of Protected Species (ICES, 2020b), which presented data on bycatch of elasmobranchs from 2018, found that Gulper shark, Leafscale gulper shark and Portuguese dogfish were all bycaught in bottom trawl fisheries. For Leafscale gulper shark, the bottom trawl bycatch rate (number of specimens observed per day at sea) in the oceanic Northeast Atlantic was 0.094. For Gulper shark, highest bycatch rates from bottom trawls were in the western Mediterranean Sea and the Aegean-Levantine Sea, both at 0.071. For Portuguese dogfish, highest bycatch rates from bottom trawl were 0.113 in the Greenland Sea. Based on the evidence presented, including the species slow recovery rates, it is likely that mobile bottom contact gear will affect the presence and distribution of the Gulper shark, Leafscale gulper shark and Portuguese dogfish features due to the associated bycatch risk.
Activity from demersal trawling and demersal seines within West of Scotland NCMPA occurs at relatively low levels and over a very limited spatial scale, and existing management is in place which already restricts the activity permitted within the site (see Section 3.2.1 above). However, the protected mobile species features of the site, as described above, are highly sensitive to demersal mobile gear activity.
Given the evidence above, the impacts from demersal mobile gears (including demersal trawls and demersal seines) alone within West of Scotland NCMPA at current levels of activity carry a risk of hindering the restoration of the Leafscale gulper shark/Gulper shark, Portuguese dogfish, Roundnose grenadier and Orange roughy features, and maintaining the Blue ling feature, such that the quality and quantity of their habitat and the composition of their population are maintained or restored. Accordingly, Scottish Ministers conclude that demersal mobile gear alone are capable of impacting the protected features and, at current levels, would or might hinder the achievement of the conservation objectives of the NCMPA.
3.3.4 Impacts of anchored nets on mobile species features
Orange roughy were only targeted using specialised bottom trawling techniques and are not commercially targeted with other gear types (FeAST, 2013), however, the species has also been recorded as bycatch in other fisheries. In the northwest Atlantic, there are records of Orange roughy caught in gillnets, with the vast majority of these at depths greater than 500 m and 800 m (96% and 92%, respectively; Kulka et al. 2001). In gillnet sets below 500 m, 0.26% of these caught Orange roughy (Kulka et al. 2001). In comparison, Orange roughy was caught in 0.49% of otter trawls below 500 m (Kulka et al. 2001). Although there is a zero TAC in place for Orange roughy, based on the evidence above, static nets may pose a risk to the presence and distribution of the species, due to the associated bycatch risk.
Although Roundnose grenadier were previously only targeted using mobile bottom contact gears in the west of Scotland area, the species can be taken using gillnets (e.g., in Canada; Simpson et al. 2011). Although there is a zero TAC in place for Roundnose grenadier, static nets may therefore pose a risk to the presence and distribution of the species, due to the associated bycatch risk.
Blue ling is landed as bycatch in Norwegian longline and gillnet fisheries targeting ling, tusk, and saithe (ICES, 2019b). However, landings from these gear types have been small since 2000 (Lorance, 2020) (ICES, 2020d). One gillnetter in the area of Hatton and Rockall Banks in 2006 caught 19 tonnes of Blue ling (Bensch et al. 2009). Trammel nets deployed between 1-25 m depth off Norway have also caught the species (Vea Salvanes, 1986) and Blue ling are bycaught in the monkfish tangle net fishery that operates to the west of Scotland (STECF, 2006). In the area to the northwest and west of Rockall, Blue ling comprised 5% of catches in 2006 (compared with 16% for the target monkfish species; STECF, 2006). At George Bligh Bank and Lousy Bank, Blue ling accounted for around 8% and 12% of the total catches, respectively. However, a high proportion of these catches were discarded due to spoilage, as Blue ling deteriorate very quickly, even with short-soak times, due to their soft-flesh. Discards were around 60% at Rockall and George Bligh Banks, although only 12% at Lousy Bank. Blue ling were previously bycaught in deep-water gillnet fisheries targeting Leafscale gulper sharks and Portuguese dogfish (Hareide et al. 2017; STECF, 2006), however this fishery has now ceased. Only minimal bycatch of Blue ling, comprising 1% of total catch, occurred in deep-water crab gillnet fisheries operating to the west of Scotland, again with high levels of discards (40%; Hareide et al. 2017; STECF, 2006). Based on the evidence available, there is a risk that the presence and distribution of Blue ling would be impacted by static nets, either as a target species or as bycatch.
Leafscale gulper shark were previously targeted in Scotland using gillnets or tangle net hybrids (Hareide et al. 2017; STECF, 2006). These fisheries have now ceased, however bycatch still occurs and the long soak times and discards of nets from gillnet fisheries are known to increase bycatch mortality (Hareide et al. 2005). There are records of Leafscale gulper shark being bycaught in monkfish tangle net fisheries in the area to the west of Scotland from observer data (STECF, 2006). At Rosemary Bank and to the northwest and west of Rockall, deep-water sharks comprised 1% of total catches, mainly comprising Leafscale gulper shark, of which 6% to 11% were discarded. Similarly, Leafscale gulper sharks are bycaught in deep-water crab gillnet fisheries on Rosemary Bank. However, deep-sea sharks comprised less than 1% of total catches, with 11% of the Leafscale gulper sharks being discarded (STECF, 2006). In a survey to retrieve lost gillnet gear in the Rockall and Porcupine Bank areas, 6,209 kg of Leafscale gulper shark were recorded from 150 gillnets/tangle nets at depths of 1,000-1,100 m in the South Porcupine area, with only 7 kg from 350 nets between 650-800 m in the SE Rockall area (Rihan et al. 2005). Over 70% of the Leafscale gulper sharks from the South Porcupine area were decayed. In terms of the selectivity of nets, only Leafscale gulper sharks with lengths in excess of 85 cm were found to be retained in retrieved nets with 160 mm mesh size (Rihan et al. 2005). Based on the evidence available, there is a risk that the presence and distribution of Leafscale gulper shark would be impacted by static nets, due to the associated bycatch risk.
Gulper shark were previously targeted in Scotland using gillnets or tangle net hybrids (Hareide et al. 2017; STECF, 2006). These fisheries have now ceased, however, bycatch still occurs targeting other species and the long soak times and discards of nets from gillnet fisheries are known to increase bycatch mortality (Hareide et al. 2005). In a study by (Moura et al. 2018) off Portugal, one Gulper shark specimen was found bycaught in the trammel net fishery targeting anglerfish in the 300-400 m depth range, however no survival information was available. Based on the evidence available, there is a risk that the presence and distribution of Gulper shark would be impacted by static nets, due to the associated bycatch risk.
Similar to the other shark species, Portuguese dogfish were previously targeted in Scotland using gillnets or tangle net hybrids (Hareide et al. 2017; STECF, 2006). These fisheries have now ceased, however, bycatch still occurs and the long soak times and discards of nets from gillnet fisheries are also known to increase bycatch mortality (Hareide et al. 2005). In a survey to retrieve lost gillnet gear in the Rockall and Porcupine Bank areas, 240 kg of Portuguese dogfish were recorded as being caught in 150 gillnets/tangle nets retrieved from depths of 1,000-1,100 m in the South Porcupine area (Rihan et al. 2005). This is much lower than the records of Leafscale gulper shark mentioned above, which is likely to be due to depleted stocks of Portuguese dogfish. Moura et al. (2018) found that off Portugal, the trammel net fishery targeting anglerfish had a very low impact on deep-water shark populations, presumably due to the species preferring deeper depths. Bycatch was recorded as <5% by weight of the total catch in 98% of the hauls at depths <600 m. The largest proportion of deep-water sharks caught (by weight and number) consisted of Portuguese dogfish, with 29 females and 1 male caught during 4 hauls in 400-500 m depth at the top of an underwater knoll. Where information on survival was available, 81% were in “poor” condition, i.e. dead, or nearly dead, or had no body movement. In the case of Portuguese dogfish, all three available specimens were classed as being in this “poor” condition category. Based on the evidence available, there is a risk that the presence and distribution of Portuguese dogfish would be impacted by static nets, due to the associated bycatch risk.
Overall there is potential for impacts on the protected mobile species features from anchored nets. It is worth noting that activity using these gear types within West of Scotland NCMPA occurs at relatively low levels and over a very limited spatial scale. Existing management is in place which already restricts the activity permitted within the site (see Section 3.2.1 above).
Given the evidence above, the impacts from anchored nets alone within West of Scotland NCMPA at current levels of activity carry a risk of hindering the restoration of the Leafscale gulper shark/Gulper shark, Portuguese dogfish, Roundnose grenadier and Orange roughy features, and maintaining the Blue ling feature, such that the quality and quantity of their habitat and the composition of their population are maintained or restored. Accordingly, Scottish Ministers conclude that anchored nets alone are capable of impacting the protected features and, at current level, would or might hinder the conservation objectives of the NCMPA.
3.3.5 Impacts of anchored lines on mobile species features
Orange roughy were only targeted using specialised bottom trawling techniques and the species is not commercially catchable by other gear types such as longlines (FeAST, 2013). For example, there were no catches of Orange roughy in 4,998 longlines sets monitored by fisheries observers between 1991 and 2000 in the Northwest Atlantic (Kulka et al. 2001). Therefore, this species is not considered further in this section.
As Roundnose grenadier are not attracted to the odour of baits, they can only be caught by trawl, rather than longlines (Priede, 2018). Jørgensen (1995) for example, didn’t record any catches of Roundnose grenadier in longlines, despite being present in large numbers in bottom trawls off west Greenland. Therefore, this species is not considered further in this section.
Blue ling are caught both as a target species and as bycatch in longline fisheries, including around Rockall and the Hatton Bank (Clark, 2006; Gordon, 2003; ICES, 2019c, 2019b, 2020c; Lorance, 2020). In the Porcupine Bank and Seabight, 597 kg (2.12% of total catch) of Blue ling were caught across 20 deep-water commercial longline deployments, with the peak catch rate occurring at 700-1,100 m (Clarke et al. 2001b). Another study found that longlines deployed in the Rockall Trough caught larger specimens of Blue ling compared to trawls (Kelly et al. 1998). From three longline sets on the Hatton Bank, catches of Blue ling ranged from 6% to 10.2% of total catch by weight (Stene and Buner, 1991 cited in Gordon, 2003). In another longline survey on the Hatton Bank at depths of 600 to 1800 m, the proportion of Blue ling caught from 67 deployments was 7.05% (by weight), compared to 1.4% from trawl (Gordon, 2003). Based on the evidence available, there is a risk that the presence and distribution of Blue ling would be impacted by static gears, either as a target species or as bycatch.
Leafscale gulper shark has previously been targeted by Irish longline, Norwegian longline and Portuguese longline fisheries, which resulted in a rapid decline in stocks (OSPAR, 2010e, 2010d). Although there is now a zero TAC in place, there remains a risk of accidental bycatch in longline fisheries and evidence shows that catch rates can be relatively high for the species. AZTI survey data in the Bay of Biscay using a former commercial deep-water shark longline (for which the number of hooks was reduced), found that Leafscale gulper sharks were caught at a rate of almost 20 kg per hook per minute between 2016 and 2018 (ICES WGEF, 2020). Individuals were more frequently caught in the bottom sections of the longline compared to the floating sections. Although the black scabbardfish longline fishery off Portugal is known to be concentrated on fishing locations where the proportion of Leafscale gulper shark catch is low (Veiga et al. 2013), data collected between 2009 to 2018 showed that the relative occurrence of Leafscale gulper sharks varied between 17% and 100%, depending on year, haul, vessel and location (ICES WGEF, 2020). From a study of three longline sets on Hatton Bank, catches of Leafscale gulper shark ranged from 15.8 to 46.2% of total catch by weight (Stene and Buner, 1991 cited in Gordon, 2003). In another longline survey on the Hatton Bank, the proportion of Leafscale gulper shark caught by longlines was 25.97% (by weight) from 67 deployments, compared to 0% by trawls (Gordon, 2003). In the Rockall Trough, evidence shows that longlines and trawls catch the same size ranges of the species (Kelly et al. 1998).
In a scientific tagging survey off Spain, Rodríguez-Cabello and Sánchez (2017) found that Leafscale gulper sharks could survive being bycaught on deep-water bottom longlines when the soaking time was restricted to 2-3 hours and lines were hauled back at very slow speeds (0.4-0.5 m/s). 1.2% of Leafscale gulper shark were dead when brought on board, with a further number being in ‘poor’ condition; the total ‘at vessel mortality’ being reported as 18.9% for this species. This species had the highest vitality rate, with 37.3% in good condition and 43.8% in moderate condition. Three out of nine Leafscale gulper sharks died within 3-10 weeks after release, however, whilst the others survived until the tags were released (45-120 days). Although this paper found that at-vessel mortality was lower than expected for deep-water sharks (i.e. <10%), post-release mortality over short and relatively long periods was sometimes high. Leafscale gulper shark was found to have the highest survival rate of all the deep-water sharks sampled (> 66%). It is worth noting however that these fishing practices are different to those normally used by commercial vessels. Research into the survival rates of Centrophorus spp. (this family includes Leafscale gulper shark and Gulper shark) taken on demersal longline gear (Wilson et al. 2009) have shown that, if handled appropriately before being released (without using automatic de-hooking gear), individuals have a high rate of survival. Another study on survival rates of Centrophorus sp. bycaught in demersal longlines in the Gulf of Mexico however found that the at-vessel mortality rate was 30.8% and the 24 hour post-release mortality rate was 83.0% (±16.0) (Talwar et al. 2017). None of the sharks exhibited correct orientation or regular, sustained swimming behaviours during the caged monitoring period underwater. Soak times were 3.5 hours and longline were hauled at a rate of 0.3 m/s. An earlier demersal longline study found similar at vessel mortality rates for Centrophorus sp. (29.41%) and data indicated that post-release predation <200 m from the surface had also occurred (Brooks et al. 2015). This predation, likely to be from pelagic sharks, therefore presents an additional risk to any individuals released. Based on the evidence presented above for Leafscale gulper sharks and Centrophorus spp., post-release mortality poses a key risk to the species. Therefore, the presence and distribution of Leafscale gulper sharks may be impacted by static gears, based on this associated bycatch risk.
Gulper shark have previously been targeted by longline fisheries and their abundance was estimated to have declined 80-95% from baseline, based on data from a target longline fishery for deep-water sharks in the north of Portugal from 1990-2004 (OSPAR, 2010c, 2010d). Although there is now a zero TAC in place, there remains a risk of accidental bycatch in longline fisheries. The 2020 report by the ICES Working Group on Bycatch of Protected Species (WGBYC; ICES, 2020c), which collated data on bycatch of elasmobranchs, found that Gulper shark was bycaught in longline fisheries. Highest bycatch rates (specimens per day at sea observed) were in the Azores at 0.019. Based on the evidence presented above and the information on survival rates for Centrophorus spp., post-release mortality poses a key risk to the species. Therefore, the presence and distribution of Gulper sharks may be impacted by static gears, based on this associated bycatch risk.
Portuguese dogfish have been targeted by Irish longline, Norwegian longline and Portuguese longline fisheries, which resulted in a rapid decline in stocks (OSPAR, 2010e, 2010d). Although there is now a zero TAC in place, there remains a risk of accidental bycatch in longline fisheries. AZTI survey data in the Bay of Biscay using a former commercial deep-water shark longline (for which the number of hooks was reduced), found that Portuguese dogfish were more frequently caught in the bottom sections of longlines compared to the floating sections (ICES WGEF, 2020). From a study of three longline sets on Hatton Bank, catches of Portuguese dogfish ranged from 1.6% to 17.7% of total catch by weight (Stene and Buner, 1991 cited in Gordon, 2003). In another longline survey on the Hatton Bank at depths of 600 to 1800 m, the proportion of Portuguese dogfish caught from 67 deployments was 17.16% (by weight), compared to 10.9% from trawl (Gordon, 2003). Although the deep-water black scabbardfish longline fishery off Portugal is known to operate at locations where Portuguese dogfish have lower abundances (Veiga et al. 2015, WD, cited in ICES WGEF, 2020), data collected between 2009 to 2018 showed that the relative occurrence of Portuguese dogfish was between 33 and 100% (ICES WGEF, 2020). Although these rates varied by haul, year, vessel and location, high numbers of specimens were consistently recorded in some places. In the Rockall Trough, evidence shows that longlines and trawls catch the same size ranges of the species (Kelly et al. 1998). In a scientific tagging survey off Spain, Rodríguez-Cabello and Sánchez (2017) found that 4.5% of Portuguese dogfish were dead when brought on board after being bycatch in deep-water bottom longlines. However, a further number of specimens were in ‘poor’ condition, increasing the at vessel mortality to 38.6%. Only 6.8% of Portuguese dogfish were in good condition, and 54.5% were in moderate condition. Two out of four Portuguese dogfish died immediately after release. Although this paper found that at-vessel mortality was lower than expected for deep-water sharks (i.e. <10%), post-release mortality over short and relatively long periods was sometimes high. It is worth noting however that these fishing practices are different to those normally used by commercial vessels. Based on the evidence above, there is a risk that the presence and distribution of Portuguese dogfish may be impacted by static gears, due to the associated bycatch risk.
Despite the potential for impacts on the protected mobile species features from anchored lines, activity using these gear types within West of Scotland NCMPA occurs at relatively low levels and over a very limited spatial scale. Existing management is in place which already restricts the activity permitted within the site (see Section 3.2.1 above).
Given the evidence above, the impacts from anchored lines alone within West of Scotland NCMPA at current levels of activity carry a risk of hindering the restoration of the Leafscale gulper shark/Gulper shark, and Portuguese dogfish features, and maintaining the Blue ling feature, such that the quality and quantity of their habitat and the composition of their population are maintained or restored. Anchored lines are unlikely to pose a significant risk at current levels of activity on the Roundnose grenadier and Orange roughy features, however. Accordingly, Scottish Ministers conclude that anchored lines alone are capable of impacting the protected features Leafscale gulper shark/Gulper shark, Portuguese dogfish, and Blue ling and, at current levels, would or might hinder the conservation objectives of the MPA.
3.3.6 Impacts of pelagic fishing on mobile species feature
Orange roughy have previously solely been targeted in the west of Scotland area using specialised bottom trawling techniques (FeAST, 2013), however, the species is known to feed on bentho-pelagic prey (Gordon and Duncan, 1987). Furthermore, the species can be caught by pelagic gear, for example the Faroese fleet’s fishery for Orange roughy uses semi-pelagic trawls (ICES, 2020c) and in other parts of the world mid-water trawls are also used (Bensch et al. 2009). Post-larval growth in Orange roughy is thought to occur in the mesopelagic, with active foraging at 700-800 m depth (Shephard et al. 2007). Spawning aggregations can also form into dynamic plumes, extending 200 m off the seabed (Branch, 2001). Although there is a zero TAC in place for Orange roughy, based on the evidence above, pelagic fishing gear may affect the presence and distribution of the species due to the associated bycatch risk at all life-stages.
Although the Roundnose grenadier is typically a bottom-dwelling, demersal fish, there are records of the species being caught in pelagic nets fished at depths between 1,000 and 2,000 m and 270 -1,440 m above the seafloor in the Denmark Strait (Haedrich, 1974). In the Rockall Trough, one study caught only small numbers of Roundnose grenadier between 3 and 60 m above the seabed in pelagic trawls (Merrit et al. 1986). The species is known to feed on pelagic prey, which descends through the water column during their daytime diel vertical migration and concentrates at the sea floor (Mauchline and Gordon, 1991). Juveniles are also thought to feed bentho-pelagically (Priede, 2019). Roundnose grenadiers may therefore play an important role in the transfer of food energy from the pelagic to the deep sea floor (Haedrich, 1974). Roundnose grenadier are thought to only exhibit vertical migrations to a few hundred metres above the seabed to intercept their prey during the day, remaining on the sea floor at night (Atkinson, 1995 cited in Priede, 2019). This pelagic behaviour appears to be rare, or only for short time periods (Mauchline and Gordon, 1991), however it does put the species at risk of being bycaught by pelagic fisheries. Furthermore, pelagic fisheries may pose an indirect threat to Roundnose grenadier, by the removal of pelagic prey species upon which Roundnose grenadier rely. Although there is a zero TAC in place for Roundnose grenadier, based on the evidence above, pelagic fishing gear may affect the presence and distribution of the species due to the associated bycatch risk at all life-stages.
Blue ling are a demersal fish and there is no evidence of the species being caught in pelagic nets, either as bycatch or as a target species. The species is therefore not considered further in this section.
Leafscale gulper shark are found at or near the seabed on continental slopes at depths of 230-2400 m, however the species has also been reported from the upper 1,250 m of oceanic water, well above the seabed in ocean depths of around 4,000 m (OSPAR, 2010e). Tagging studies have shown that the species can travel over long distances (maximum estimated at 990 nm), with some individuals making large slow vertical displacements throughout the water column, lasting several hours (Rodríguez-Cabello et al. 2016; Rodríguez-Cabello and Sánchez, 2014). In some instances, individuals travelled in midwater thousands of metres above abyssal plains. This species is therefore at risk of being bycaught by pelagic fisheries. Furthermore, the species also appears to be highly migratory and exhibits size, maturity and sex related distribution patterns (Clarke et al. 2001a, 2005; Moura et al. 2014). Within the NE Atlantic, there is a lack of juveniles and pregnant females recorded, but late stage pregnant females appear to segregate from the general population in other areas with pupping occurring in various locations, including potentially off Ireland (Priede, 2019). This puts the species at risk from fisheries impacts over a wide area, with an increased risk of bycatch occurring when the species is migrating. In an experimental midwater drifting longline fishing survey for black scabbardfish off the Canary Islands, Leafscale gulper shark were the most captured species, with 170 individuals caught over twenty hauls (one with a line containing around 500 hooks and the second with a line containing 5000 hooks; Freitas et al. 2018) The 2020 report by the ICES Working Group on Bycatch of Protected Species (ICES, 2020b), which collated data on bycatch of elasmobranchs, found that Leafscale gulper shark were bycaught in pelagic trawl fisheries. Bycatch rates (number of specimens observed per day at sea) were highest in the Celtic Seas and were recorded as 0.111. Although there is a zero TAC in place for Leafscale gulper shark, based on the evidence above, pelagic fishing gear may affect the presence and distribution of the species due to the associated bycatch risk at all life-stages.
Gulper shark have been recorded at depths from 98 to 1700 m, suggesting that they may use the water column (Priede, 2019). Although there is no reliable information on migrations or the pupping grounds of Gulper shark, pregnant females appear to segregate from the rest of the population along the outer edge of continental shelves and in canyons (Priede, 2019). This poses a greater risk for the species, as there is a risk of bycatch occurring when over a wider area. In an experimental midwater drifting longline fishing survey for black scabbardfish off the Canary Islands, 10 Gulper sharks were caught from 20 hauls, one with a line containing around 500 hooks and the second with a line containing 5000 hooks (Freitas et al. 2018). The 2020 report by the ICES Working Group on Bycatch of Protected Species (ICES, 2020b), which collated data on bycatch of elasmobranchs, found that Gulper shark were bycaught in pelagic trawl fisheries. Bycatch rates (number of specimens observed per day at sea) were highest in the Celtic Seas and were recorded as 0.333. Although there is a zero TAC in place for Gulper shark, based on the evidence above, pelagic fishing gear may pose a risk to the presence and distribution of the species, due to the associated bycatch risk at all life-stages.
Portuguese dogfish are one of the deepest living sharks and are known to occur on or near the seabed, from 700 –1900 m, in the area to the west of Scotland (Priede, 2019). There is evidence of the species exhibiting vertical migration and females are known to move to shallower waters to give birth (500-1000 m), increasing risks of interactions with fisheries (Clarke et al. 2001a; Girard and Du Buit, 1999; Moura et al. 2014; OSPAR, 2010f; STECF, 2006). Mature females have been found dominating some catches, for example. This species is known to feed on fish and squid, including Roundnose grenadier, indicating bentho-pelagic foraging (Mauchline and Gordon, 1983, cited in Priede, 2019), putting the species at risk of being bycaught by pelagic fisheries. Furthermore, pelagic fisheries may pose an indirect threat to Portuguese dogfish, by the removal of pelagic prey species upon which Portuguese dogfish rely. The species is not thought to be highly migratory as different maturity stages and sizes are found in the same geographical areas, so it is likely that the species can complete its life cycle within the same area (Moura et al. 2014). Recolonization from neighbouring areas will therefore be extremely slow, with recovery likely to take longer than 25 years (OSPAR, 2010d), similar to that of the other deep-water shark species discussed here. Although there is a zero TAC in place for Portuguese dogfish, based on the evidence above, pelagic fishing gear may pose a risk to the presence and distribution of the species, due to the associated bycatch risk at all life-stages.
Despite the potential for impacts on the protected mobile species features from pelagic fishing, activity using these gear types within West of Scotland NCMPA occurs at relatively low levels and over a very limited spatial scale. Existing management is in place which already restricts the activity permitted within the site (see Section 3.2.1 above).
Given the evidence above, the impacts from pelagic fishing alone within West of Scotland NCMPA at current levels of activity would not hinder restoring the Leafscale gulper shark/Gulper shark, Portuguese dogfish, Roundnose grenadier, Blue ling and Orange roughy features, such that the quality and quantity of their habitat and the composition of their population are maintained or restored. Accordingly, Scottish Ministers conclude that pelagic fishing alone is capable of impacting the protected mobile species features but would not hinder the achievement of the conservation objectives of the site at current levels of activity.
3.4 Part B Conclusion
The assessment of fishing pressures on the protected features of West of Scotland NCMPA has indicated that demersal trawling, demersal seines and anchored net/line activities would or might hinder the achievement of the conservation objectives of the site.
However, there are a number of exceptions for some of the protected features. Anchored net/lines, at current activity levels has indicated that for orange roughy and roundnose grenadier, burrowed mud, offshore sands and gravels and offshore deep-sea muds the activity would not hinder the achievement of the conservation objectives for the for West of Scotland NCMPA.
As such the Scottish Ministers concludes that management measures are required to restrict demersal trawling, demersal seines, and anchored nets/lines within West of Scotland NCMPA. Section 5 contains further details on these measures.
Scottish Ministers conclude that the remaining fisheries activities (pelagic fishing), when considered in isolation and at current levels, will not hinder the achievement of the conservation objectives for West of Scotland NCMPA.
Contact
Email: marine_biodiversity@gov.scot
There is a problem
Thanks for your feedback