Quality prescribing for antidepressants: guide for improvement 2024 to 2027
Antidepressant prescribing continues to increase in Scotland with one in five adults receiving one or more antidepressant prescriptions in a year. This guide aims to further improve the care of individuals receiving antidepressant medication and promote a holistic approach to person-centred care.
5. Reducing and stopping antidepressants
Quick links
- Standard reduction: SSRI, SNRI, TCA, Other antidepressants
- Difficulty withdrawing SSRI/SNRI
- Significant difficulty/fear of withdrawing SSRI/SNRI
Discontinuation
All classes of antidepressants can cause discontinuation/withdrawal symptoms, especially when stopped abruptly. Therefore, this advice is intended to provide prescribers and individuals with a range of options to appropriately support and enable successful antidepressant reduction and discontinuation.
Discontinuation/withdrawal effects may also occur to a lesser extent when doses are missed or reduced. It is, however, unknown what the specific incidence and prevalence is – as this can vary by individual antidepressant (e.g. more commonly occurs with paroxetine and venlafaxine), duration of treatment, the condition being treated and study design – studies have indicated that that up to 17% of people receiving placebo and up to 15-56% of people receiving different antidepressants may be affected. [133], [134],[135], [136] It is also estimated that 3% of people may experience severe withdrawal effects.[136] However, some individuals may be more sensitive to withdrawal than others, and unfortunately, it is difficult to know who will or will not experience discontinuation/withdrawal effects.
Discontinuation symptoms
The term ‘discontinuation symptoms’ is used to describe symptoms experienced on stopping medicines that are not drugs of dependence, although there are important semantic differences in the terms ‘discontinuation’ and ‘withdrawal’ symptoms – the latter implying addiction, the former does not.[18] While the distinction is important for precise medical terminology, it is irrelevant when it comes to personal experiences and how an individual may describe their signs and symptoms.
The optimum rate of taper to prevent withdrawal effects is unknown.[22],[23] Therefore, the prescriber and individual should discuss and agree on the most appropriate approach to reducing the dose and reviewing progress – for some this will mean ‘low and slow reductions’. This will vary depending on individual’s needs, circumstances, age, clinical condition and other comorbidities being treated, as well as the duration of antidepressant treatment. However, some individuals who stop antidepressant treatment for depression may experience a depressive relapse. A recent large robust trial by Lewis et al.[137] assessed the risk of relapse for people who indicated that they were ready to stop their antidepressant which they had taken for two years or more. During a 52 week follow up period 39% of people continuing antidepressant treatment experienced a depressive relapse, with 56% of those discontinuing treatment. Of the latter group 39% subsequently restarted antidepressant treatment
Considerations for reduction and/or stopping an antidepressant
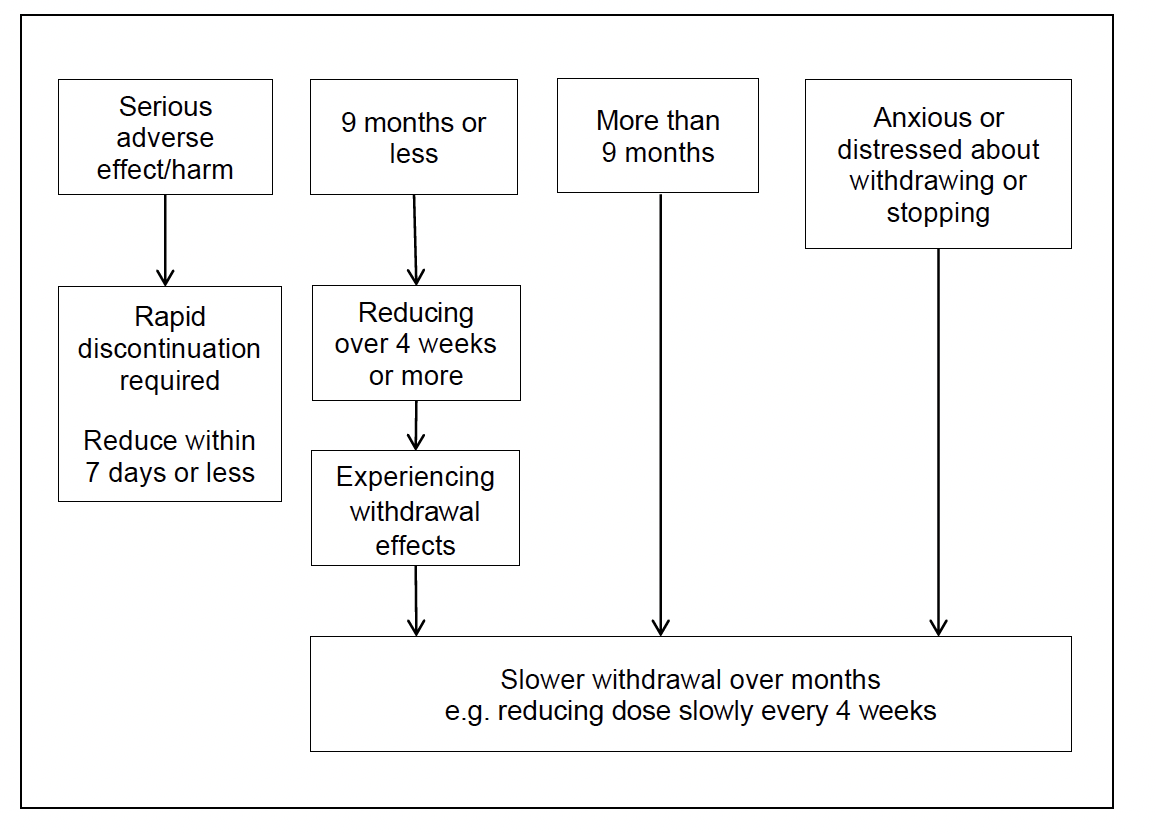
Note: Nine months was assessed as being an appropriate point when an individual may have completed a six-month course of treatment for first episode of depression and account for the time required to receive an effective antidepressant and dose. For example, first antidepressant partially effective at four weeks, then required to switch to alternative antidepressant, dose titration where appropriate, then six-month course for people achieving remission.
The strategy for reducing/stopping antidepressants should be guided and informed by the individual’s preferences and needs. Consider the clinical situation when reviewing and discussing reducing/stopping antidepressants. Encourage individuals to discuss stopping their antidepressants with their prescriber before doing so.
By discussing and planning withdrawals, the most appropriate rate of reduction can be agreed and planned with the individual, according to their preferences and needs:
- If experiencing serious adverse effects/harms - may require rapid discontinuation within seven days or less (Table 3).
- Completed a nine month or less course of antidepressant treatment e.g. with first episode of moderate to severe depression – reduce over a minimum of four weeks. Some individuals may need a slower reduction e.g. four weekly stepped reductions. (Shorter courses of antidepressant treatment may be less likely to be associated with discontinuation/withdrawal effects. However the rate of reduction should be guided by the individual’s preferences and needs.)
- Completed a longer course (nine months or more) of antidepressant treatment, and/or a history of recurrent depression or anxiety. Reducing and tapering the dose at a slower rate over months may be more appropriate, e.g. for people with anxiety disorders who have responded to antidepressants and received long-term treatment a minimum of three months or longer, tapered reduction is recommended.
- Anxious about reducing/withdrawing antidepressants or history of experiencing discontinuation effects. Reducing and tapering the dose at a slower rate over months may be more appropriate.
Where people experience significant or unbearable withdrawal effects during reduction, increasing back to the previous dose that did not cause withdrawal symptoms, and after stabilising, considering a slower rate of reduction may help.
Adverse effect |
Drugs |
Symptoms/Signs |
|---|---|---|
Serotonin syndrome (very rarely occurs) |
SSRI, SNRI, clomipramine, moclobemide, and other medicines e.g. triptans, tramadol, fentanyl, etc. |
Mild (individual may/may not be concerned): insomnia, anxiety, nausea, diarrhoea, hypertension, tachycardia, hyperreflexia Moderate (causes distress): agitation, myoclonus, tremor, mydriasis, flushing, diaphoresis, low fever (<38.5°C) Severe (medical emergency): severe hyperthermia, confusion, rigidity, respiratory, coma, death |
QTc interval prolongation |
Citalopram, escitalopram, TCAs, and other medicines e.g. quinine, methadone, antipsychotics, antibiotics etc. |
ECG changes in QTc interval |
Note: Serotonin syndrome, for more detail see Buckley et al. 2014[138] and Isbister 2007[139] QTc prolongation is of concern as it is associated with ventricular tachycardia and sudden cardiac death, see Kallergis et al. 2012.[51]
Assess the individual’s readiness to reduce and/or stop
It is important that an individual’s motivation and readiness for reduction and/or discontinuation is adequately assessed. Where agreed a tailored dose reduction should be planned, and where implemented, regularly reviewed. Signposting or referral for interventions to support changes to prescribing should also be considered, including psychosocial or psychological interventions.
What is the risk-benefit balance of continuing current antidepressant dose?
Example considerations may be:
- balancing anticholinergic effects versus neuropathic pain control
- reducing the signs and symptoms of the condition it was prescribed for
or the
- need to stop the antidepressant due to increased risks e.g. cardiovascular disease, QTc prolongation risk or a newly diagnosed condition
Has the individual completed the planned and agreed course or trial of treatment?
For example, in relation to depression, is the individual experiencing residual symptoms such as sleep issues, irritability and ruminating, motivation (have they managed to do something they like to do) and do they have plans for the future (moving on from depressive episode)? These factors may indicate that an individual is suitable to consider reducing and stopping their antidepressant after completing an appropriate course of treatment. This can be six months for the first episode of depression or 12 or 24 months of treatment, depending on the number of depressive episodes and relapses.
Discontinuation/withdrawal symptoms: These may begin on average within two days (up to five days) after stopping and occasionally following dose reduction or missed doses. Generally, these symptoms subside within seven to ten days,[18],[20] but may include a wide range of symptoms which can vary in intensity, depending on which antidepressant is being stopped (Tables 4 and 5). For some people these symptoms are mild and self-limiting, however, others may experience severe or prolonged discontinuation or withdrawal symptoms e.g. flu-like symptoms, electric shocks (brain zaps), vivid dreams, dizziness or diarrhoea. Unfortunately, the optimum rate of taper to prevent discontinuation/withdrawal symptoms is unknown.[22],[23]
|
Symptoms |
|---|---|
Systemic, cardiac effects |
Flu-like symptoms*, dizziness/drowsiness*, tachycardia (fast heart rate)*, impaired balance, fatigue, weakness, headache, dyspnoea (breathlessness) |
Sensory |
Paraesthesia (burning, prickling sensation)*, electric shock–like sensation (“brain zaps/body zaps”)*, sensory disorders, dysesthesia (abnormal unpleasant sensation e.g. burning, itching), itch, tinnitus, altered taste, blurred vision, visual changes |
Neuromuscular |
Muscle tension*, myalgia (muscle pain)*, neuralgia (nerve pain)*, agitation*, ataxia (lack of muscle co-ordination)*, tremor, akathisia (inner restlessness, urgent need to constantly move, inability to stand/sit still) |
Vasomotor |
Perspiration*, flushing*, chills*, impaired temperature regulation |
Gastrointestinal |
Diarrhoea*, abdominal pain*, anorexia, nausea, vomiting |
Sexual |
Premature ejaculation*, genital hypersensitivity* |
Sleep |
Insomnia, nightmares, vivid dreams, hypersomnia (excessive sleepiness) |
Cognitive |
Confusion*, disorientation*, amnesia*, reduced concentration |
Affective |
Irritability, anxiety, agitation, tension, panic, depressive mood, impulsivity, sudden crying, outbursts of anger, mania, increased drive, mood swings, increased suicidal thoughts, derealization, depersonalization |
Psychotic |
Visual and auditory hallucinations |
Delirium |
Typically only with tranylcypromine |
Adapted from Henssler et al 2019[130] and Haddad et al. [140] Symptoms in bold occur more frequently. *Serotonin related
Antidepressant class |
Most commonly associateda |
Common symptomsb |
Occasional symptomsb |
|---|---|---|---|
SSRI, Clomipramine (TCA) |
Paroxetine |
Flu-like symptoms (chills, myalgia, excess sweating, nausea, headache), ‘shock-like’ sensations, dizziness exacerbated by movement, insomnia, excess (vivid) dreaming, irritability, crying spells |
Movement disorders, concentration, memory difficulties |
SNRIs |
Venlafaxine |
Same as above, due to serotonin effects |
Same as above |
TCAs |
Amitriptyline Imipramine |
Flu-like symptoms, insomnia, excess dreaming Anticholinergic rebound – more common in older adults: headache, restlessness, diarrhoea, nausea and vomiting |
Movement disorders, mania, cardiac arrhythmias |
Other |
Mirtazapinec |
Anxiety, panic attacks, insomnia, irritability, nausea |
- |
Other |
Agomelatine |
- |
No discontinuation symptoms have been reportedd |
Other |
Trazodone |
- |
Rarely SSRI type withdrawalse |
Other |
Vortioxetine |
- |
No discontinuation symptoms have been reportedf |
a. Although most commonly associated with the listed medicines, other medicines in the group may cause similar symptoms.
b. Symptoms: As individuals may or may not experience discontinuation/withdrawal symptoms, and the intensity and range of symptoms may vary by individual, people may experience or identify symptoms not listed above.
c. Limited data: mirtazapine case studies, see Cosci et al. 2017.[141]
d. At time of writing no case reports in literature. Agomelatine rarely used.
e. See Haddad et al. 2001[136] and Otani et al. 1994[142] for more detail.
f. Adapted from and informed by Maudsley and Psychotropic Drug Directory. Vortioxetine rarely used.
Standard reduction approaches
Standard reduction approaches may be appropriate for individuals taking antidepressants that have no past history of distressing withdrawal, and no particular fear of withdrawing and/or stopping antidepressants over four to six weeks.
Review and reduce dose every one to four weeks, or as guided by the individual’s needs and/or preferences. However, reducing by one step every four weeks may be more practical for individuals due to their carer, family and work commitments, as well as for collecting prescriptions and enabling appropriate face-to-face or telephone review follow-up.
Selective serotonin re-uptake inhibitors (SSRIs) [143]
Due to the long half-life, the following can be stopped at standard daily doses: citalopram 20mg, escitalopram 10mg, fluoxetine 20mg and sertraline 50mg per day. However, individuals may prefer or require a slower reduction with lower doses.
SSRIs |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
Step 6 |
|---|---|---|---|---|---|---|
Citalopram |
40mg |
30mg |
20mg |
10mg |
Stop |
- |
Escitalopram |
20mg |
15mg |
10mg |
5mg |
Stop |
- |
Fluoxetine |
40mg |
30mg* |
20mg |
10mg** |
Stop |
- |
Fluvoxamine |
300mg |
200mg |
100mg |
50mg |
Stop |
- |
Sertraline |
200mg |
150mg |
100mg |
50mg |
25mg |
Stop |
Paroxetine† |
40mg |
30mg |
20mg |
10mg |
5mg†† |
Stop |
Steps: the rate of withdrawal will vary with individual needs e.g. weekly to four weekly reductions for some.
All doses are single daily doses
*Alternate day dosing 40mg/20mg
**Alternate day dosing with 20mg capsule, or consider using fluoxetine liquid
† Some individuals may require to be switched to an alternative SSRI if experiencing significant withdrawals, see below.
†† Half a 10mg tablet
Serotonin and noradrenaline re-uptake inhibitors (SNRIs)
Most individuals will be able to slowly withdraw and discontinue duloxetine and venlafaxine without any adverse effects. Where individuals experience discontinuation/withdrawal effects after stopping, it may be appropriate to restart the antidepressant at the previous dose and frequency for seven days then switch to a long-acting SSRI if interactions and contra-indications allow.
SNRI |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
Step 6 |
|---|---|---|---|---|---|---|
Duloxetinea |
120mg |
90mg |
60mg |
30mg |
Stop |
- |
Venlafaxine MRb |
300mg |
225mg |
150mg |
75mg |
37.5mg |
Stop |
Venlafaxinec |
150mg twice daily |
150mg morning 75mg night |
75mg twice daily |
37.5mg twice daily |
Stopd |
- |
Steps: the rate of withdrawal will vary with individual needs e.g. weekly to four weekly reductions for some, or longer and slower reductions for others.
Note: Venlafaxine 300mg daily used as example, as individuals on higher doses are usually under the care of community mental health teams who should be involved in decisions to reduce or withdraw.
a. BNF only has 60mg dose listed for treatment of major depressive order. Duloxetine SmPC (data sheet) quotes up to 120mg daily. However, there is no clinical evidence suggesting that individuals not responding to the initial recommended dose may benefit from dose up-titrations.[144]
b. If receiving modified-release (MR) preparations as split dose e.g. twice daily, please consider that MR preparations are intended as once daily preparations.
c. Some individuals may have a preference for reducing the night-time or morning dose first.
d. If needed venlafaxine 37.5mg MR daily could be used for another step before stopping.
Tricyclic antidepressants (TCAs)
Frail and/or older adults may require and need slower reduction to minimise the risk of cholinergic rebound (nausea, vomiting, headache, restlessness). Therefore, slow reduction over longer than six weeks, or months, may be needed for some individuals depending on their preference and/or needs.
TCAs |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
Step 6 |
Step 7 |
Step 8 |
|---|---|---|---|---|---|---|---|---|
Amitriptylinea |
150mg |
100mg |
50mg |
Stop |
- |
- |
- |
- |
Amitriptylinea,b |
150mg |
125mg |
100mg |
75mg |
50mg |
25mg |
10mgc |
Stop |
Lofepramined |
210mg |
140mg |
70mg |
35mge |
Stop |
- |
- |
- |
a. The same reduction schedule could be advised for:
- Clomipramine
- Dosulepin (dothiepin)
- Doxepin
- Imipramine
- Nortriptyline
- Trimipramine
b. Older adults and some individuals may require reductions using smaller dose increments to minimise the risk of adverse withdrawal effects/harms.
c. Dosulepin and doxepin not available as 10mg dose, therefore, consider if necessary, using 25mg capsules on alternate days, then stop.
d. If dose is split morning and night, consider reducing and stopping morning dose first, and then continuing reduction with nighttime dose.
e. Tablets are less suitable for halving as they have a film coating. If necessary, a 35mg dose can be given using lofepramine 70mg/5ml oral suspension.
Other antidepressants and monoamine oxidase inhibitors (MAOIs)
Other antidepressants/MAOIs |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
Step 6 |
Step 7 |
|
|---|---|---|---|---|---|---|---|---|
Agomelatine |
50mg |
25mg |
Stop |
- |
- |
- |
- |
|
Mirtazapine |
45mg |
30mg |
15mga |
Stop |
- |
- |
- |
|
Trazodone |
300mgb |
250mg |
200mg |
150mg |
100mg |
50mg |
Stop |
|
Vortioxetine |
20mg |
10mg |
Stop |
- |
- |
- |
- |
|
Isocarboxazidc |
Morning |
60mg |
50mg |
40mg |
30mg |
20mg |
10mg |
Stop |
Moclobemided |
Morning |
300mg |
300mg |
150mg |
150mg |
Stop |
- |
- |
Night |
300mg |
150mg |
150mg |
Stop |
- |
- |
- |
|
Phenelzinec |
Morning |
30mg |
30mg |
30mg |
15mg |
15mg |
15mg |
Stop |
Afternoon |
30mg |
15mg |
15mg |
15mg |
Stop |
Stop |
- |
|
Night |
30mg |
30mg |
15mg |
15mg |
15mg |
Stop |
- |
|
Tranylcyprominec |
Morning |
30mg |
20mg |
10mg |
Stop |
- |
- |
- |
Steps: the rate of withdrawal will vary with individual needs e.g. weekly to four weekly reductions for some.
a. Some individuals may find the 15mg dose more sedating than higher doses due to greater antihistamine effects at lower doses.
b. For higher doses consider reducing at each step by 50mg. However, clinical need and/or individual preferences may require larger reduction steps e.g. 100mg.
c. Isocarboxazid, phenelzine and tranylcypromine inhibit monoamine oxidase A and B for up to two weeks after stopping. Consider risk of interactions for two weeks after stopping.
d. Moclobemide is a reversible inhibitor of monoamine oxidase A.
Difficulty withdrawing SSRI/SNRI
For individuals with difficulty withdrawing SSRI/SNRIs, or those who are fearful of withdrawing, switching to a longer half-life (longer acting) SSRI (e.g. fluoxetine) may enable a smoother reduction in antidepressant blood levels. This may be of use, especially for individuals who are having difficulty stopping short half-life antidepressants: paroxetine, venlafaxine or duloxetine. Venlafaxine and duloxetine act as SSRIs at low dose.
Convert to long-acting SSRI, then reduce and stop[143]
Reduce the total daily dose in a stepwise fashion to: paroxetine 20mg, venlafaxine 75mg, duloxetine 30mg daily (see SSRI and SNRI). Then convert to an approximate dose equivalent* of fluoxetine, citalopram or sertraline (Step 1), using standard capsules, tablets or liquid. Switch by taking the last dose of paroxetine/venlafaxine/duloxetine today, and then starting the new dose of fluoxetine tomorrow at the same time of day.[18,20] Then stabilise on that dose for three to seven days then stop, as per previously reported.[145],[146] For example, duloxetine 30mg daily changed to fluoxetine 20mg daily and continued for three to seven days then stopped. However, some individuals may prefer or need slower reductions.
SSRI |
Half-life (T1/2) |
Time to almost complete elimination (five half-lives) (hours, unless specified) |
|---|---|---|
Citalopram |
35 hours |
7.3 days |
Escitalopram |
30 hours |
6.25 days |
Fluoxetine [Norfluoxetine]* |
4-6 days [4-16 days] |
20-24 days [20-80 days] |
Paroxetine** |
24 hours |
5 days |
Sertraline |
26 hours |
5.4 days |
*Active metabolites
**Paroxetine and venlafaxine are associated with a greater incidence of withdrawal effects. There are mixed reports of discontinuation symptoms with Duloxetine.
SNRI |
Half-life (T1/2) |
Time to almost complete elimination (five half-lives) (hours, unless specified) |
|---|---|---|
Duloxetine |
12 hours |
60 hours (2.5 days) |
Venlafaxine [Desmethylvenlafaxine]* |
5 hours [11 hours] |
1 day [2.3 days] |
*Active metabolites
Alternate day dosing – half-life of antidepressants
The half-life of an antidepressant determines if it is appropriate for alternate day dosing. In general, most medication effects will be considered negligible/insignificant after three half-lives and will be eliminated from the individual`s system after five half-lives, but there are exceptions to this.
Citalopram, escitalopram, fluoxetine and sertraline all have long half-lives (see Table 10 above). If active metabolites are considered this can be up to 48 days (3 x 16 days) for fluoxetine. Therefore, alternate day (48 hour) dosing is possible with these drugs.
Venlafaxine and duloxetine are inappropriate for alternate day dosing due to their short half-life. For some people it may be appropriate to switch from ordinary release twice daily dosing of venlafaxine to once daily modified release (MR) preparations, to allow further reduction prior to stopping. For example, venlafaxine 37.5mg twice daily to 75mg MR once daily, then reducing to 37.5mg MR daily before stopping.[148]
Paroxetine causes more withdrawal/discontinuation effects than sertraline even though their half-lives are comparable.[149] This is due to complex pharmacokinetics.[150] The high affinity of paroxetine for muscarinic receptors can lead to cholinergic rebound, contributing to withdrawal/discontinuation syndrome.[151]
Daily dose |
Step 1* |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
Step 6 |
|
|---|---|---|---|---|---|---|---|
Duloxetine 30mg Or Paroxetine 20mg Or Venlafaxine MR 75mg (37.5mg twice daily) |
To any of the SSRIs in Step 1 |
Fluoxetine 20mg |
20mg alternate days |
20mg every third day |
Stop† |
- |
- |
Citalopram 20mg |
10mg |
10mg alternate days |
Stop |
- |
- |
||
Sertraline 50mg |
25mg |
12.5mg (half a 25mg tablet) |
Stop |
- |
- |
||
Fluoxetine liquida,b,c (20mg in 5ml) |
16mg (4ml) |
12mg (3ml) |
8mg (2ml) |
4mg (1ml) |
Stop |
Steps: the rate of withdrawal will vary with individual needs e.g. weekly to four weekly reductions for some.
† Consider risk of interactions for two weeks after stopping
a. Some community pharmacies may not stock 1ml graduated 5ml oral syringes, but they can order if given advance notice.
b. Citalopram 40mg/ml drops and escitalopram 20mg/ml drops are not recommended due to the difficulty with accurately measuring small doses with drops.
c. Sertraline liquid is not recommended as it is unlicensed in the UK, and individuals may experience oral numbness on their tongue and mouth due to the anaesthetic effects of non-tablet formulations.
*Approximate dose equivalents and switching considerations:[18,49,134]
Due to inter-patient variability and differing half-lives, this means that these are approximate dose equivalents, not exact equivalence.
- The drug and dose equivalents can never be exact and should be interpreted considering your clinical knowledge and the individual’s needs.
- Drug interactions and drug-disease interactions should be considered
- Fluoxetine liquid may be required for a few individuals that require or prefer a slower reduction at weekly to four weekly intervals.
Significant difficulty or fears withdrawing SSRI/SNRI
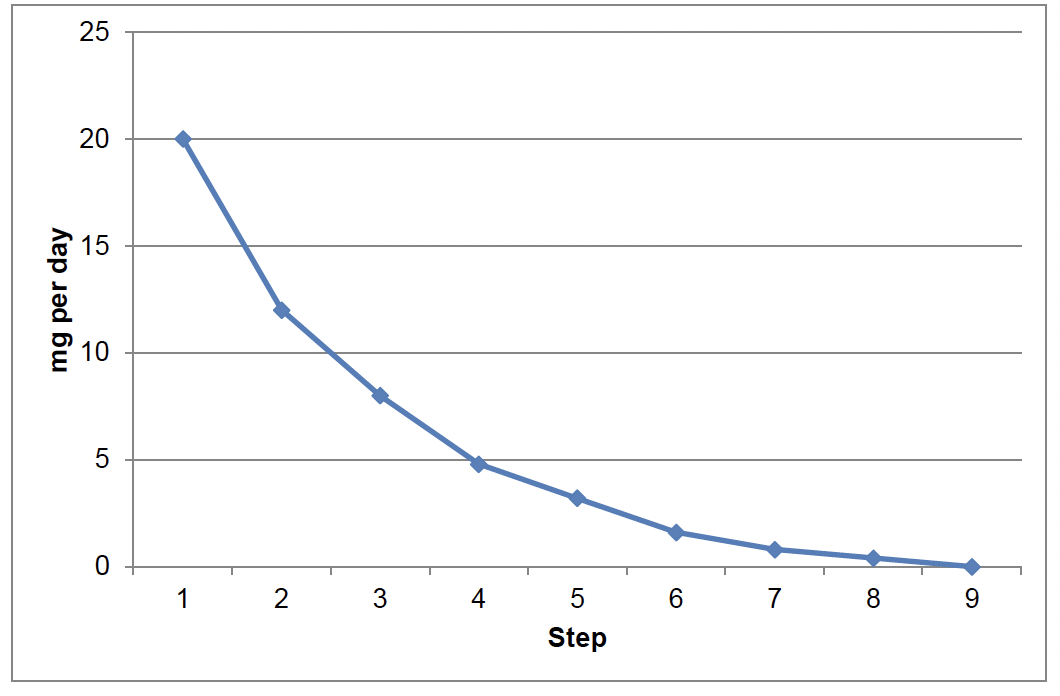
For a very small minority of individuals, slower graduated reduction may be appropriate. For example, where standard reduction and/or discontinuing/withdrawing SSRI/SNRI has been unsuccessful. This approach will help flatten the reductions in plasma drug concentrations at lower doses ( Chart 3).
First, reduce current antidepressant to standard dose as per SSRI or SNRI. Then convert to an approximate dose equivalent of fluoxetine 20mg/5ml liquid.
Fluoxetine 20mg is approximately dose equivalent* to:
- Citalopram 20mg
- Escitalopram 10mg
- Fluvoxamine 50mg
- Paroxetine 20mg
- Sertraline 50mg
- Duloxetine 30mg
- Venlafaxine 75mg
*Approximate dose equivalents and switching considerations:[18,49]
Due to inter-patient variability and differing half-lives, this means that these are approximate dose equivalents, not exact equivalence.
- The drug and dose equivalents can never be exact and should be interpreted considering your clinical knowledge and the individual’s needs.
- Drug interactions and drug-disease interactions should be considered prior to any switch in therapy.
For example, if switching paroxetine 20mg daily to fluoxetine 20mg daily, or paroxetine 10mg daily to fluoxetine 8mg daily (step 3 below). Switch by taking the last dose of paroxetine today, and then start the new dose of fluoxetine tomorrow at the same time of day.[18,20] Agree on an appropriate rate of reduction e.g. weekly or monthly and time for face-to-face or telephone review follow-up.
Step |
mg/d |
ml/d |
Step down Difference (mg) |
|---|---|---|---|
1 |
20 |
5 |
- |
2 |
12 |
3 |
8 |
3 |
8 |
2 |
4 |
4 |
4.8 |
1.2 |
3.2 |
5 |
3.2 |
0.8 |
1.6 |
6 |
1.6 |
0.4 |
1.6 |
7 |
0.8 |
0.2 |
0.8 |
8 |
0.4 |
0.1 |
0.4 |
9 |
Then stop |
0 |
0.4 |
Note:
- Steps: the rate of withdrawal will vary with individual needs e.g. weekly to four weekly reductions for some.
- Citalopram 40mg/ml and escitalopram 20mg/ml liquid are not recommended due to the difficulty with accurately measuring small doses.
- Sertraline liquid is not recommended as it is unlicensed in the UK, and individuals may experience oral numbness on their tongue and mouth due to the anaesthetic effects of non-tablet formulations
- Table 13 adapted with consideration of Horowitz et al,[152] Ruhe et al,[153] Selvaraj et al,[154] and The Maudsley Prescribing Guidelines in Psychiatry 14th edition.[18]
Contact
Email: EPandT@gov.scot
There is a problem
Thanks for your feedback