Genomic medicine strategy 2024 to 2029
Our strategy for transforming genomic medicine across Scotland from 2024 to 2029.
9. Timely and equitable access to genetic testing
We want to ensure that genomic medicine in Scotland supports diagnosis and access to the right treatment and management, at the right time, for the right person with a national genomic test directory that is harmonised with clinical management pathways.
Background
Test directories serve as a backbone to any genomic medicine service providing information to clinical teams about tests available, and where they can provide clinically meaningful information. They represent a dynamic catalogue and require continuous review to ensure that older testing methods are phased out, or supplemented, and that new tests are developed and validated for adoption in a timely manner to meet clinical need.
Where we are now
As service commissioners, NSD currently maintain two genomic test directories: the Scottish Cancer Test Directory and the Scottish Rare and Inherited Disease Test Directory.[16, 17] These online directories detail the tests available to clinicians in Scotland as well as the referral criteria and turn-around times (TATs). The Rare and Inherited Disease Test Directory has benefited from over £8 million in investment since 2017, as part of the Bridge to a Scottish Strategy for Genomics project, including support for clinical exome sequencing across all four Scottish genomic laboratories and a whole exome sequencing (WES) service which is delivered in collaboration with the University of Edinburgh.
Within the Scottish Cancer Test Directory it is recognised that there are significant gaps, with some tests recommended as part of clinical guidelines or to accompany medicines accepted for use by the Scottish Medicines Consortium (SMC), which are not yet available. Across Scotland, a number of genomic tests are sent externally to NHS or commercial laboratories elsewhere in the UK or abroad because the testing technology and capacity does not yet exist either in Scotland or in the UK as a whole.
There are, however, gaps remaining in the availability of genomic testing in Scotland that we need to address urgently. As part of this strategy, we want to ensure that people in Scotland have access to the required genomic testing. In doing so we will continue to collaborate with our counterparts across the UK to maintain flexibility to send extremely rare tests to specialised centres where necessary.
Where we want to be
We will ensure that the Scottish test directories are comprehensive, taking account of developments in genomic medicine and staying responsive to clinical need. To support our longer-term service development these directories will be aligned as far as possible with both the other nations of the UK and relevant international standards to ensure that people in Scotland have access to the same medicines and standards of care.
Horizon scanning and the identification of future trends, technologies and tests
There are a wealth of resources, organisations and professional bodies that provide advance information about new tests and targets: from drug discovery, pre-clinical development and clinical trials to changes in clinical management guidelines around diagnosis, prognosis or prevention.
Robust horizon scanning for the SSNGM should not replicate this work but resource, expertise and funding are required to consolidate advance information on new tests and targets with sufficient notice to ensure engagement with HCPs, service users and the genomic laboratories. In doing so, we will work to strengthen the SSNGM as the ‘front door’ for genomic testing in Scotland to encourage early engagement and a collaborative approach encompassing academia, industry and the third sector.
This process will be further supported by the Access to New Medicines Horizon Scanning Advisory Board (HSAB) which has been established to identify and analyse new medicines currently in licensing and Health Technology Assessment (HTA) pipelines that are due to be considered by the SMC for routine access within the following 18-24 months.
Robust test assessment
Test directories require a robust review framework in place to evaluate and optimise existing tests as well as assess novel tests, tools and technologies identified through horizon scanning. The SSNGM has established a Scottish Genomics – Test Advisory Group (SGTAG) for both cancer and rare and inherited conditions.
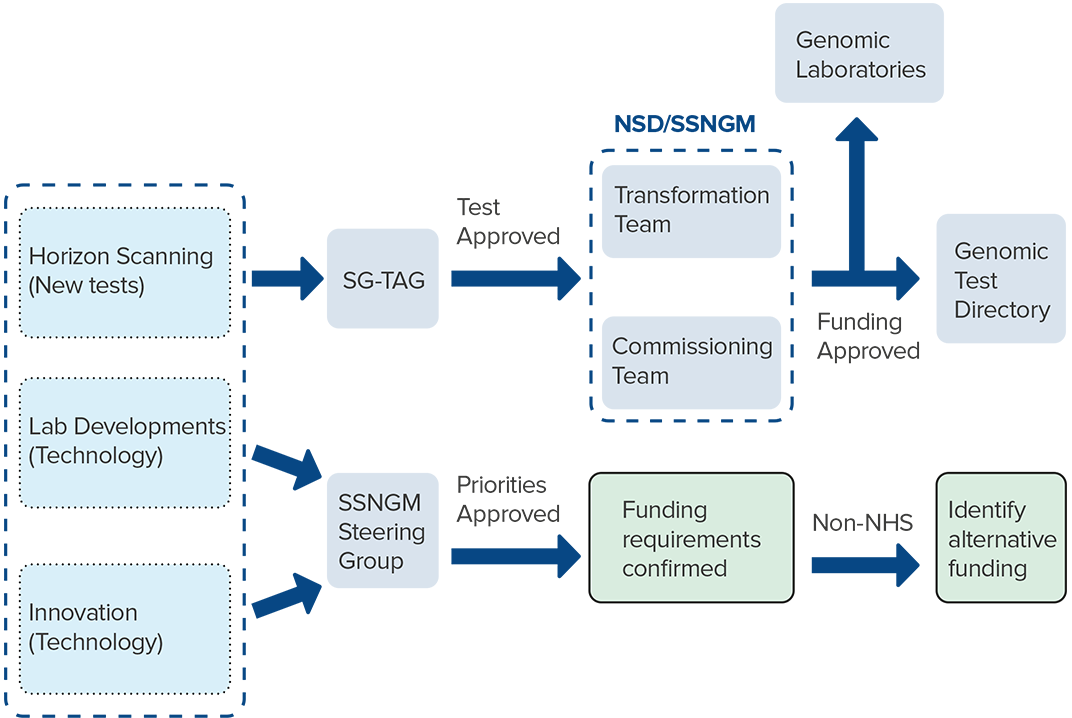
The purpose of this process is to ensure that decisions relating to the approval of new tests added to the test directories are based on the best available evidence regarding efficiency and efficacy. SGTAG will make recommendations to NSD and the SSNGM on the clinical and analytical validity, meaningful clinical impact, patient outcomes and best delivery model for tests.
For the assessment of more complex tests and technologies, particularly in relation to rare conditions or cancers with more complex genomic profiles, we will look for additional support and health economics input via the Scottish Health Technology Group (SHTG) as well as our academic partners, recognising holistic health economic assessments as key to understanding and evidencing the impact genomic medicine has on the recovery, renewal and transformation of our NHS services.
Presently, existing funding models are a major factor in the delay between identification of new tests and their adoption into the national test directories. We will develop a sustainable funding model which is responsive and supports the timely and national adoption of tests and technologies approved under SGTAG, including real-time delivery in line with SMC approval of medicines with associated genomic testing in collaboration with different partners.
Ensuring equitable access
As part of the SSNGM transformational programme, the Scottish test directories will be mapped against those offered in England, Wales and Northern Ireland to identify gaps and divergence. It is important to ensure that people in Scotland receive as equitable a standard of care and access to testing as the rest of the UK and that available testing is aligned as far as possible but is also responsive to clinical needs in Scotland. Where the test directories do differ in terms of test methods, turn-around times or referral criteria, there should be a clinical and scientific rationale as to why this is the case, and clear communication with HCPs, clinical networks and service users. Beyond our close neighbours in the UK we will also continue to follow the advancement of genomic medicine around the world, including engaging with life sciences companies of all sizes to ensure that the Scottish test directories remain responsive and fit for purpose. We also recognise the importance of genomic information and tumour profiling not only in supporting better care but to support clinical pathways where access to UK-wide and international clinical trials and experimental therapies are embedded within standard of care.
Expansion of genomic testing
We will work with the Scottish Cancer Network, regional cancer networks and the managed clinical networks to identify testing gaps within the cancer test directory, ensure SGTAG review and plan for timely implementation of tests approved for adoption. We will also deliver a broader range of testing technologies across cancer and rare and inherited conditions and determine the most cost effective way of doing so. This will include the analysis of circulating tumour DNA (ctDNA) and large panel Next-Generation Sequencing (NGS) for cancer and Whole Genome Sequencing (WGS) where these technologies will deliver clinical benefit. Key to the delivery of these technologies will be collaborative working with clinical teams, service users and academic, industry and third sector partnerships.
Integration with clinical pathways
Alongside the development of the Scottish test directories we need to maintain close collaboration and ongoing dialogue between the SSNGM and genomic laboratories with the clinical teams and networks responsible for maintaining clinical pathway resources. This is to ensure that information within the test directories is aligned and up-to-date and is clearly signposted to both clinical teams and service users.
Clinical pathway resources currently exist in different formats and across a range of platforms, depending on the teams responsible for their development and maintenance. The Right Decision Service, which started out as an innovative new approach trialled by the Digital Health and Care Innovation Centre, is evolving as a go-to point for national clinical pathways and clinical decision support.[18] The service has now transitioned into business as usual activity with Healthcare Improvement Scotland, with a clear vision to scale up its use.
The Scottish Cancer Network has developed a series of clinical management pathways for breast cancer, neurological and lung cancer on the platform. Additional pathways will be added in due course. The SSNGM will look to integrate, where possible, with the Right Decision Service to signpost relevant test information, test directories and support resources within these clinical pathways. As part of the Right Decision Service, there is also scope to develop clinical decision support tools with targeted information, linked to the test directories and test requesting in a way that is consistent with wider national efforts.
The SSNGM recognises the time and effort involved in developing clinical pathways for inclusion within the Right Decision Service and will also collaborate with clinical teams, networks and service users across Scotland to integrate links or signposting to the genomic test directories into clinical pathway guidance where appropriate. We will also look to strengthen the Scottish Clinical Genetics Forum to allow them to advise on required changes in practice and improve clinical pathways in response to rapid changes in genomic knowledge and, particularly, on areas of cross-over around the management of germline (inherited) variants.
Delivery models
While genomic testing for clinical use must be delivered within accredited laboratories, there is scope for partnership with other organisations around the analysis of clinical genomic data and the reanalysis of data as new knowledge develops both nationally and internationally. The Whole Exome Service (WES) Service for developmental delay currently offered by NHS Lothian, the University of Edinburgh and the Edinburgh Parallel Computing Centre (EPCC) currently sits within the research environment (see Case Study 19.1) but has demonstrated considerable value and resulted in new diagnoses that, for affected patients and their families, have been life-changing. We are committed to translating work such as this into accredited services and exploring their potential for other clinical indications and testing technologies.
What will this mean for patients and the people of Scotland?
Timely access to the right test will shorten the time people have to wait for their genomic results. This can allow an appropriate treatment plan to be started sooner that can improve people’s lives, giving better outcomes from quicker intervention.
Contact
Email: holly.ennis@gov.scot
There is a problem
Thanks for your feedback